Breakthrough Catalyst Transforms Methane Emissions at Room Temperature: A Game Changer for the Environment!
2024-11-21
Author: Sophie
Introduction
In a significant leap towards combating climate change, researchers have unveiled a groundbreaking catalyst capable of recycling methane emissions at room temperature—an achievement that could revolutionize the way we address one of the most potent greenhouse gases associated with fossil fuels.
The Role of Methane in Environmental Impact
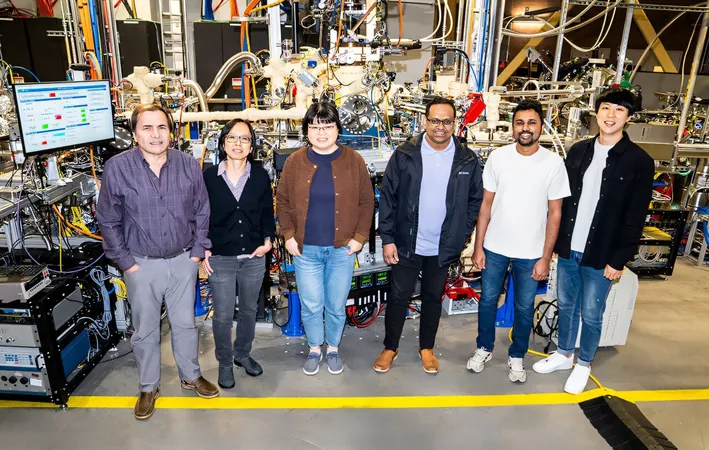
Natural gas, a crucial energy source for heating, cooking, and electricity generation in the U.S., is primarily composed of methane, making it a leading contributor to greenhouse gas emissions. As scientists explore innovative ways to convert methane into useful fuels and products, a recent study led by Brookhaven National Laboratory's team from the U.S. Department of Energy (DOE) has produced a low-cost catalyst that shows remarkable efficiency without the need for the high temperatures typically required for activation.
Details of the Catalyst
Published in the October 15, 2024 issue of the journal ACS Nano, the catalyst combines magnesium oxide nanoparticles with copper oxide, forming a structure that is not only effective but also economically scalable for commercial use. This innovative approach marks a step forward, as many traditional catalysts demand temperatures exceeding 500K (approximately 440°F), which raises both energy costs and operational complexity.
Milestone in Methane Conversion
Finding a catalyst that ensures effective methane conversion at such moderate temperatures is a milestone in this field," stated Arephin Islam, the lead author of the research. The technology revolves around activating magnesium oxide, which, in its bulk form, falls short for methane conversion, but when finely distributed and coupled with the right metal oxides, performs extraordinarily well.
Research Techniques Used
To better understand this catalyst's mechanics, researchers deployed advanced techniques including ambient-pressure X-ray photoelectron spectroscopy (AP-XPS) and scanning tunneling microscopy (STM). These state-of-the-art methods allowed scientists to observe chemical interactions and surface characteristics in real time, providing unprecedented insights into the catalyst’s reactivity.
Dual Functionality of the Catalyst
Notably, the team demonstrated that the magnesium oxide-copper oxide catalyst not merely activates methane at room temperature but efficiently breaks down its carbon-hydrogen bonds to produce ethane—an essential chemical used in manufacturing refrigerants and various fuels. The catalyst’s performance stands toe to toe with more expensive platinum-based alternatives, promising a more sustainable solution.
Potential for Carbon Dioxide Conversion
This impressive technology does not stop with methane. In another study, researchers also confirmed that the catalyst can convert carbon dioxide at room temperature, potentially turning a harmful greenhouse gas into valuable chemicals. This dual functionality could open new pathways toward mitigating climate impact and developing renewable energy sources.
Conclusion
Given the urgency of climate action, this catalyst represents a significant advancement. By transitioning from harmful emissions to useful products at lower costs and temperatures, this research is paving the way for greener industrial applications and more effective carbon mitigation strategies. As the world grapples with the climate crisis, innovations like this could be the key to achieving substantial reductions in greenhouse gas emissions—make sure to keep an eye on what comes next in this field!







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)