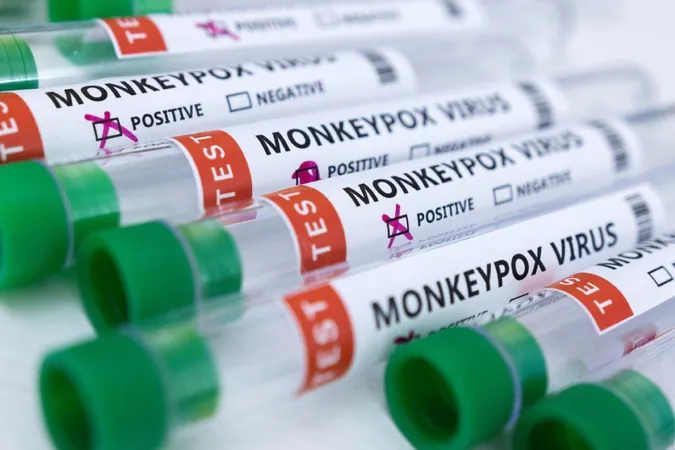
Breakthrough: China Prepares to Launch Clinical Trials for Mpox Vaccine!
2024-12-13
Author: Jacques
Introduction
In a significant step towards combating mpox, China's top drug regulatory authority has greenlit clinical trials for a groundbreaking vaccine developed by Sinopharm, a leading domestic pharmaceutical company. This development signals a move closer to addressing public health concerns associated with the virus, which has garnered attention due to its alarming spread globally.
Vaccine Development
The innovative vaccine is the collaborative effort of the Beijing Institute of Biological Products, overseen by Sinopharm, alongside the National Institute for Viral Disease Control and Prevention, part of the Chinese Center for Disease Control and Prevention. In a statement released Thursday, the company confirmed that the vaccine candidate demonstrated both safety and efficacy during preclinical testing, effectively triggering immune responses against mpox across various animal models, including nonhuman primates.
Significance of the Vaccine
This vaccine represents the ingenuity of Chinese scientists as it is independently developed and holds complete proprietary intellectual property rights,” remarked Sinopharm. The company anticipates that this vaccine will be instrumental in controlling and preventing mpox outbreaks within China.
Clinical Trials and Process
The process for a vaccine to be approved in China is rigorous, typically consisting of three phases of clinical trials, which can take several years, if not decades, to complete. In addition to Sinopharm’s candidate, another promising vaccine is being developed by the Shanghai Institute of Biological Products, a subsidiary also connected to Sinopharm, which received the go-ahead for clinical trials just last month.
Novel Approaches in Vaccine Research
Adding to the excitement, researchers at the Institute of Microbiology under the Chinese Academy of Sciences are exploring an entirely novel messenger RNA-based mpox vaccine. Early results from studies conducted in mice have shown promising outcomes, indicating that this method may provide another effective pathway for immunization against the virus. The institute has also established agreements with drug manufacturers to fast-track research and facilitate the market registration of their experimental vaccine.
Global Context and Impact
The need for efficient vaccination strategies is pressing. The World Health Organization declared mpox a global health emergency in August 2022 after a surge in cases across Africa, particularly in Congo, Burundi, and Uganda, with over 120 countries and regions reporting confirmed cases.
Domestic Situation in China
On the domestic front, China faced its first mpox case in June of the previous year, subsequently classifying it as a Class B infectious disease, which ranks alongside COVID-19 and HIV/AIDS in terms of public health urgency. According to the latest data from the China CDC, 46 infections were reported in September, with an additional 38 cases in October, emphasizing the importance of these vaccine developments.
Conclusion
As clinical trials commence, the hope is that China will lead the charge in mitigating the impact of mpox domestically and potentially offer solutions to countries grappling with the outbreak. Stay tuned as this critical public health story continues to unfold!





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)