Breakthrough in Bimetallic Catalysts: Researchers Achieve Atomic Precision
2025-01-08
Author: Jacob
A groundbreaking study has revealed that bimetallic particles, which consist of a noble metal partnered with a base metal, exhibit extraordinary catalytic properties, especially in selective heterogeneous hydrogenations. This research highlights how the geometric and electronic structures of these particles contribute to their unique abilities.
For effective and selective hydrogenation to occur, it is crucial that the active atoms on a catalyst engage specifically with the functional groups targeted for transformation within a substrate. The innovation in this field comes from reducing catalyst particles to nanoscale atomic clusters and single-atom alloys. This not only enhances surface dispersion, making noble metal atoms more accessible, but also transforms the electronic structure of the active sites, significantly influencing both intrinsic activity and product distributions.
In a significant advancement, a research team led by Professors Shen Wenjie and Li Yong from the Dalian Institute of Chemical Physics, in collaboration with Professors Li Weixue from the University of Science and Technology of China and Wang Yuemin from the Karlsruhe Institute of Technology, successfully engineered atomically precise active sites for hydrogenation reactions.
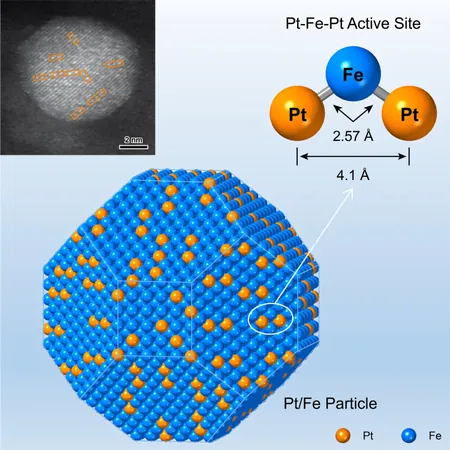
The researchers devised a new method to densely populate and accurately position isolated platinum (Pt) atoms in the form of Pt-Fe-Pt heterotrimers on α-Fe nanoparticles. This heterotrimer is created through the hydrogen reduction of a Pt-Fe2O3 particle pair, wherein a 3.3 nm Pt particle resides atop a 9.8 nm Fe2O3 particle. During the hydrogen reduction process, the iron oxides are transformed into iron, allowing for the dispersion of Pt particles into heterotrimers through surface alloying, which is essential for catalytic activity.
Moreover, the study revealed the formation pathway and coordination environment of the Pt-Fe-Pt heterotrimer. When subjected to gas-phase hydrogenation of crotonaldehyde, the heterotrimer preferentially hydrogenated the C=O bond to yield crotyl alcohol, rather than engaging the conjugated C=C bond. Astonishingly, the intrinsic rate of hydrogenation surged by a staggering 35 times, effectively overcoming the typical activity-selectivity trade-off found in hydrogenation reactions.
What makes this development even more fascinating is the recognition of a site-bond interaction pattern within the Pt-Fe-Pt heterotrimer. The left-end Pt atom acts to anchor the C=C bond, while the central Fe atom activates the C=O bond, which is subsequently hydrogenated by hydrogen atoms that are adsorbed on the right-end Pt atom.
"This study quantifies the surface catalytic reaction at the molecular level and offers a novel strategy for customizing active sites on bimetallic catalysts with atomic precision," commented Prof. Shen, marking a significant leap in the field of catalysis. This advancement not only provides insights into the fine-tuning of catalyst structures but also promises enhancements in industrial processes that rely heavily on selective hydrogenation, potentially leading to more efficient and sustainable chemical manufacturing practices.
As researchers continue to explore the vast potential of bimetallic catalysts, the implications for energy, materials science, and industrial chemistry remain profound. This breakthrough could transform approaches to catalysis, leading to innovations that might only be limited by our imagination. Stay tuned as this exciting field evolves, offering promising solutions to meet global energy challenges!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)