Breakthrough in Cancer Treatment: Revolutionary Light-Guided siRNA Delivery System Developed Using Cyanobacteria!
2024-11-26
Author: Olivia
Introduction
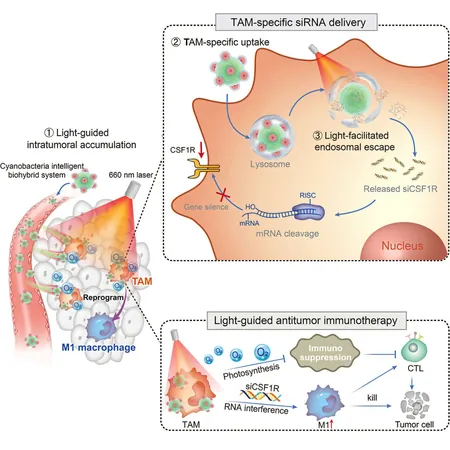
In an exciting development in the fight against cancer, researchers have unveiled an innovative light-guided biohybrid system, known as CTPA/siCSF1R, designed to revolutionize the delivery of small interfering RNA (siRNA) to tumor-associated macrophages (TAMs). This groundbreaking study, published in *Cell Reports Physical Science* on November 25, showcases a new approach that enhances the tumor microenvironment and supports more effective photoimmunotherapy techniques.
Understanding Tumor-Associated Macrophages (TAMs)
Understanding the role of TAMs is crucial in this context; these immune cells are integral to the tumor microenvironment and serve as prime targets for immunotherapy. Traditional gene therapy has faced challenges due to subpar targeting abilities, limited escape from lysosomes, and a hypoxic, immunosuppressive environment that reduces its clinical efficacy.
The Role of Cyanobacteria in siRNA Delivery
Enter the cyanobacterium Synechocystis sp. PCC6803, affectionately dubbed "cyan." This living biological carrier not only displays self-driven targeting capabilities towards tumors but also boasts phototactic properties, allowing precise drug delivery when combined with external light stimuli. This unique characteristic, alongside its ability to produce oxygen through photosynthesis, positions cyanobacteria as an exceptional vehicle for delivering nucleic acid-based drugs directly to solid tumors.
Mechanism of CTPA/siCSF1R System
The CTPA/siCSF1R system intricately combines a triblock polyamino acid (TPA) gene vector encapsulating siRNA, which is cleverly conjugated to the surface of the photosynthetic cyanobacteria. By harnessing the self-driving and phototactic properties of these tiny organisms, researchers can accurately target TAMs nestled within the tumor microenvironment.
Activation and Efficacy
When activated by light, the cyanobacteria produce reactive oxygen species that effectively disrupt lysosomal membranes, enabling the release of siRNA into the cytoplasm of TAMs. Additionally, the oxygen generated through photosynthesis not only bolsters the tumor microenvironment but also amplifies the delivery efficiency of siRNA and enhances TAM reprogramming.
Experimental Findings
Experimental findings indicate that the CTPA/siCSF1R system successfully reprograms TAMs to adopt an M1 phenotype, inciting a robust immune response characterized by the release of pro-inflammatory cytokines that effectively hamper tumor growth. Moreover, the system has exhibited excellent biosafety profiles, ensuring minimal toxicity to host organisms.
Future Implications
This promising advancement could mark a significant leap toward the development of efficient and safe nucleic acid delivery vectors, alongside improving the outcomes of tumor photoimmunotherapy strategies. With continued research and development, this innovative approach could reshape cancer treatment paradigms for years to come.
Research Leadership
Leading this pioneering work is Professor Cai Lintao from the Shenzhen Institutes of Advanced Technology (SIAT), a branch of the esteemed Chinese Academy of Sciences. The implications of this research not only inspire hope in the medical community but also paint a brighter future for patients battling various forms of cancer. Stay tuned as this story unfolds!






 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)