Breakthrough in Carbon Technology: Rocket-Inspired Reactivity Achieves Unprecedented Surface Area for Energy and Environmental Applications
2024-12-18
Author: Jacob
Research Breakthrough
In an impressive leap forward, researchers from Cornell University have successfully developed a nanoporous carbon material with an extraordinary surface area of 4,800 square meters per gram. This groundbreaking discovery, inspired by the ignition of rocket fuel, is poised to revolutionize carbon-dioxide capture technologies and energy storage systems.
Importance of Carbon Materials
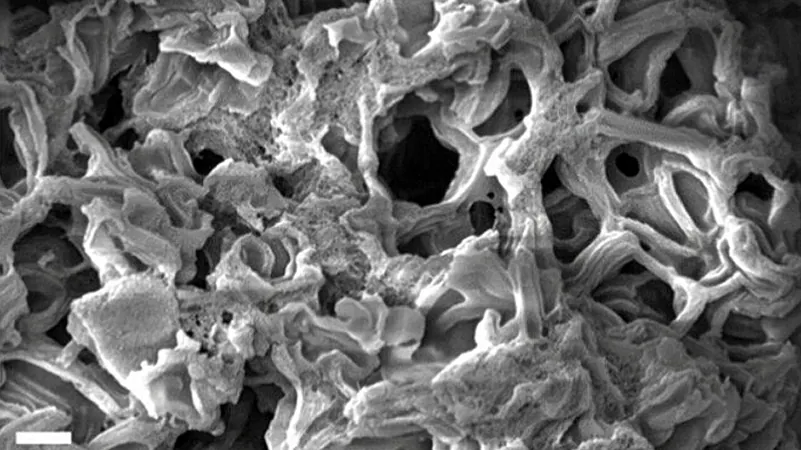
Carbon materials are essential in a variety of applications, particularly in trapping pollutants and storing electrical energy. Increasing the porosity of carbon allows for a greater surface area, which enhances its effectiveness. In this latest research, the innovative method detailed in the journal ACS Nano reveals a way to maximize surface area without sacrificing the structural integrity of the material.
Balancing Surface Area and Mass
Senior author Emmanuel Giannelis, a noted professor in the Department of Materials Science and Engineering at Cornell, emphasizes the importance of balancing surface area with the material's actual mass: "You can reach a level of porosity where it's essentially just air; the challenge lies in maintaining enough material to be functional."
Methodology of Research
The team's strategy involved the expertise of postdoctoral researcher Nikolaos Chalmpes, who applied principles of hypergolic reactions—typically utilized in rocket propulsion systems. Through careful manipulation of various parameters in these fast-paced reactions, Chalmpes aimed to synthesize carbon structures with ultra-high porosity.
Synthesis Process
The process starts with sucrose and a template material that shapes the carbon. Mixing these with specific compounds initiates a hypergolic reaction, resulting in unique carbon tubes characterized by five-membered molecular rings—quite different from the standard six-membered rings present in conventional carbon configurations. Subsequent treatment with potassium hydroxide further refines this structure, erasing less stable sections and creating a complex array of microscopic pores.
Reaction Mechanics
Giannelis elaborates, "The rapid reaction conditions prevent the system from settling into its most stable state, capturing it instead in a metastable form. This reaction speed allows us to achieve results that slow heating methods simply cannot replicate."
Performance Highlights
The research highlights not only the carbon's ability to absorb carbon dioxide but also its impressive performance compared to traditional activated carbons, absorbing nearly twice as much CO2 and achieving 99% of its total capacity in just two minutes. This makes it one of the fastest-acting sorbents available.
Energy Storage Potential
Additionally, the material shows immense potential for energy storage, producing a volumetric energy density of 60 watt-hours per liter—four times greater than commercially available options.
Broader Implications
Chalmpes remarks on the broader implications of their work: "This method provides an innovative pathway for developing carbon-based materials that can serve as sorbents, catalysts, and components in supercapacitors, especially where space efficiency is critical. Furthermore, the unique hypergolic reaction conditions open new avenues for designing and synthesizing advanced electrocatalysts."
Conclusion
This pioneering research not only expands the horizons of carbon materials science but also contributes significantly to advancements in environmental sustainability and energy efficiency. Stay tuned for further developments in this exciting field!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)