Breakthrough in CO2 Reduction: Scientists Unveil Electrolyte-Dependent Selectivity with Revolutionary MOF Catalyst
2024-09-25
Introduction
In an exciting development in the quest to combat climate change, scientists are harnessing the power of metal-organic framework (MOF) catalysts to selectively reduce carbon dioxide (CO2) into valuable products, contingent on the composition of the electrolytes used. This novel approach offers a promising new direction in CO2 utilization strategies.
Groundbreaking Study
A groundbreaking study published in Angewandte Chemie International Edition highlights how the electrolyte's makeup can significantly influence the products yielded during the electrochemical reduction of CO2. Traditionally, research has focused primarily on optimizing catalyst designs for improved selectivity, but this new work shifts some of the attention to electrolyte composition—an area previously least explored.
Research Team and Catalyst Design
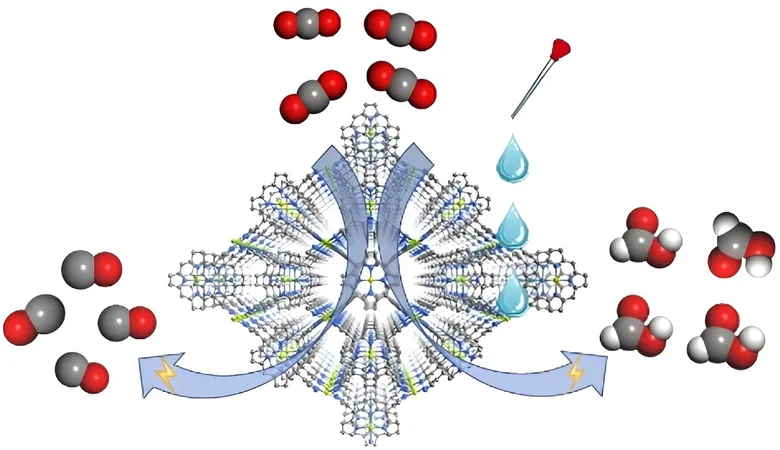
The research team, led by notable figures such as Prof. Cao Rong and Prof. Zhang Teng from the Fujian Institute of Research on the Structure of Matter under the Chinese Academy of Sciences, engineered a MOF catalyst known as FICN-8. This innovative catalyst is synthesized from copper-based ligands and pyrazolate units, resulting in a three-dimensional porous structure that enhances the accessibility of its catalytic sites.
Electrochemical Performance
Electrochemical tests demonstrated that FICN-8 possesses impressive activity for CO2 conversion, especially in a tetrabutylammonium hexafluorophosphate (TBAPF6)/acetonitrile (MeCN) electrolyte, achieving a remarkable selectivity of up to 95% for carbon monoxide (CO).
Impact of Water and TFE
However, adding water or trifluoroethanol (TFE) to the electrolyte drastically altered the product landscape; the primary reduction product transitioned from CO to formic acid (HCOOH). Strikingly, with the right concentrations—2.65 mol/L of water or 0.55 mol/L of TFE—the Faradaic efficiency for formic acid production peaked at an impressive 48%.
Mechanistic Insights
To understand the underlying mechanics of this selectivity switch, the researchers conducted various experiments, revealing key insights through kinetic isotope effect (KIE) measurements. These measurements indicated a near-identical KIE value for CO production, while formic acid formation exhibited a substantial KIE of 3.7 ± 0.7, thereby illuminating the direct involvement of protons in the path leading to HCOOH.
Theoretical Analysis
Further theoretical analyses emphasized the significance of reductive adsorption of hydride (H) on the porphyrin nitrogen site as a pivotal step in the formation of formic acid, contrasting with CO production, which appears to follow a separate mechanism not reliant on proton availability.
Conclusion and Future Perspectives
This discovery holds great potential for developing advanced electrocatalysts that not only optimize CO2 reduction but also contribute to a more sustainable approach to harnessing carbon dioxide. As worldwide efforts intensify to mitigate greenhouse gas emissions, findings like these may represent a critical step toward innovative carbon management solutions.
Stay tuned for more developments in this vital area of research that could reshape our environmental strategies!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)