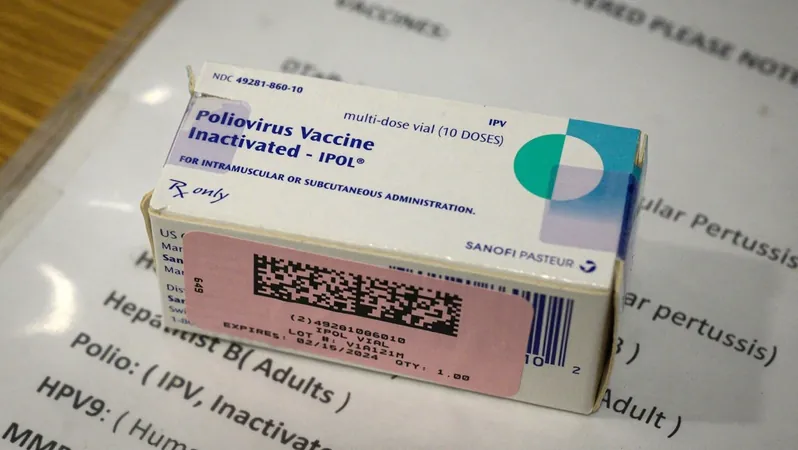
Controversy Erupts as Lawyer Demands FDA to Reassess Polio Vaccine Approval Amid Trump's Praise
2024-12-15
Author: Benjamin
Introduction
In a surprising turn of events, a lawyer connected with Robert F. Kennedy Jr., a prominent vaccine skeptic and President Donald Trump's appointed pick for the U.S. Department of Health and Human Services, has formally petitioned the U.S. Food and Drug Administration (FDA) to revoke its approval of the polio vaccine used widely in the country. This petition, filed by Aaron Siri on behalf of the Informed Consent Action Network (ICAN), a nonprofit organization that critically examines vaccine safety, raises questions amidst the backdrop of Trump's past endorsements of the vaccine.
FDA Review
The FDA confirmed that it is currently reviewing Siri’s petition but declined to comment further on the timeline for this review. If Kennedy is confirmed to lead the Department, he would ultimately oversee the FDA and may engage in its decision-making process concerning the petition. In a recent NBC News interview, Kennedy emphasized the importance of choice, saying, "People ought to have a choice, and that choice ought to be informed by the best information."
Trump's Support
Despite the petition's challenges, Trump, in a late November interview with Time magazine, reiterated his support for polio vaccination, labeling it "the greatest thing." He indicated openness to future examinations of vaccine safety for children but clarified that groundbreaking vaccines like those for polio are vital and should remain intact unless proven otherwise.
McConnell's Statement
U.S. Senate Minority Leader Mitch McConnell, a polio survivor himself, asserted the critical role of the polio vaccine. "The polio vaccine has saved millions of lives and held out the promise of eradicating a terrible disease," he remarked. "Efforts to undermine public confidence in proven cures are not just uninformed—they're dangerous."
Background of Polio Vaccine
The background of the polio vaccine is crucial in understanding the weight of this debate. Introduced in the 1950s during a time when polio outbreaks paralyzed thousands, the vaccine has significantly decreased the disease's incidence worldwide. Once responsible for over half a million global deaths annually, polio is now on the brink of eradication due to widespread vaccination efforts.
Siri's Petition
Siri's petition argues that the FDA should suspend approval of the inactivated poliomyelitis vaccine until a properly controlled double-blind clinical trial can assess its safety. However, public health experts point out that conducting placebo-controlled trials for vaccines is often considered unethical due to the potential risk of unprotected individuals contracting polio. Such trials would also undermine the ongoing efforts to maintain effective vaccination levels and herd immunity.
Expert Opinions
Dr. Paul Offit, a vaccine expert, challenged the claims made in the petition, explaining, “You’re substituting a theoretical risk for a real risk. The real risks are the diseases." Public health authorities, including the CDC, maintain that no serious adverse events linked to the use of the inactivated polio vaccine have been documented, although rare allergic reactions do occur.
Inactivated vs Oral Vaccine
The petition specifically targets the inactivated vaccine currently used in the U.S., which is injected and poses less risk of causing paralysis compared to the oral vaccine, which, while still used in some countries, was phased out in the U.S. due to safety concerns.
Polio Transmission
Poliovirus itself spreads easily via the fecal-oral route, and while the oral vaccine has historically helped in preventing disease, it poses risks if not administered widely in communities. In 2023, a notable 524 polio cases were reported due to vaccine-derived strains, a decrease from the previous year, highlighting the delicate balance required to maintain public health standards.
Conclusion
As this debate continues, the implications for public health policies and vaccination strategies remain significant, prompting questions about the future role of vaccines in the American healthcare system. Will the FDA respond favorably to Siri’s petition, or will the legacy of polio vaccination withstand this challenge? Only time will tell as this issue evolves in the political landscape.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)