Groundbreaking Atomic Force Microscopy Unveils Microtubule Defects at Unprecedented Resolution
2024-12-12
Author: Amelia
Groundbreaking Atomic Force Microscopy Unveils Microtubule Defects at Unprecedented Resolution
In an exciting breakthrough, researchers from the Nano Life Science Institute (WPI-NanoLSI) at Kanazawa University in Japan have harnessed frequency-modulated atomic force microscopy (FM-AFM) to illuminate the submolecular structure of microtubules (MTs) and expose critical defects within their lattice. This groundbreaking study, published in Nano Letters, promises to reshape our understanding of the dynamic processes governing microtubule functionality.
Microtubules are essential components of the cellular cytoskeleton in eukaryotic cells. They act as scaffolds, contributing to a variety of crucial cellular activities, including cell division, migration, and intracellular transport. Each microtubule is a cylindrical structure composed of α-tubulin and β-tubulin proteins that polymerize into dimers, forming linear protofilaments.
Historically, techniques like X-ray crystallography and cryo-electron microscopy have provided insights into MT structures. However, these methodologies require intricate sample preparation and data analysis, underscoring the demand for advanced tools capable of examining microtubule features and defects under physiological conditions.
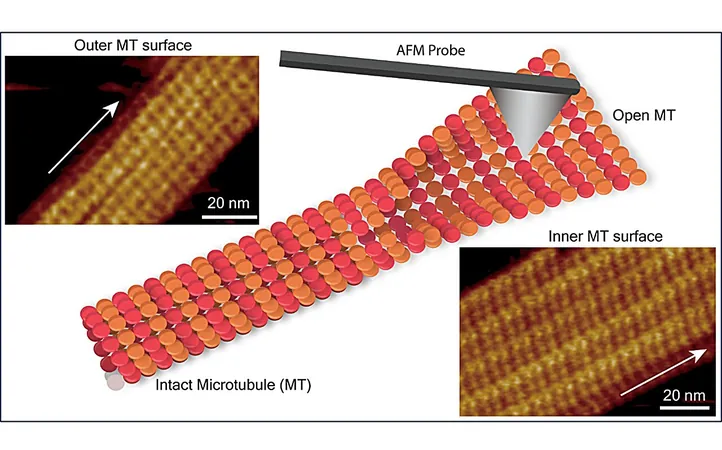
While considerable attention has been devoted to studying the outer surface of microtubules, the submolecular configuration of tubulin dimers within the inner microtubule wall remained largely uncharted territory. This inner wall interacts differently with various proteins compared to the outer wall, further highlighting the need for detailed examination.
To fill this research gap, a team led by scientists Ayhan Yurtsever, Hitoshi Asakawa, and Takeshi Fukuma employed FM-AFM to explore the structural arrangements of tubulin dimers on both the inner and outer surfaces of microtubules. Their findings revealed that the inner surface possesses a corrugated structure, while the outer surface features shallower undulations. These subtle differences are critical, as they can significantly impact microtubule stability and function.
The research uncovered that one protofilament was consistently higher in topography than its neighboring protofilament due to variations in the orientation and conformation of the αβ-tubulin heterodimers. During transitions from tubular to sheet-like structures on the inner surface, the individual tubulin monomers underwent reorientation, creating a dynamic landscape within the microtubules.
Significantly, the study identified defects characterized by missing tubulin subunits within the microtubule lattice. These structural anomalies can disrupt the arrangement of protofilaments, ultimately impairing various microtubule functions, including critical roles in cellular transport and division.
Additionally, the researchers investigated the interaction between microtubules and Taxol, a chemotherapeutic agent that specifically binds to β-tubulin subunits. Taxol stabilizes microtubules, effectively inhibiting the division and migration of cancer cells—a property that has profound implications in cancer therapy.
This innovative approach using FM-AFM not only allows for high-resolution imaging of microtubule structures but also offers insights into the molecular interactions of drugs targeting these essential components. As research continues to unravel the complexities of microtubule biology, findings like these could pave the way for the development of new therapeutic strategies against various diseases, including cancer, enhancing our ability to manipulate cellular processes with precision.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)