
Groundbreaking Combined Covid-19 and Flu Vaccine Trial Kicks Off in Brisbane!
2024-10-14
Author: Emma
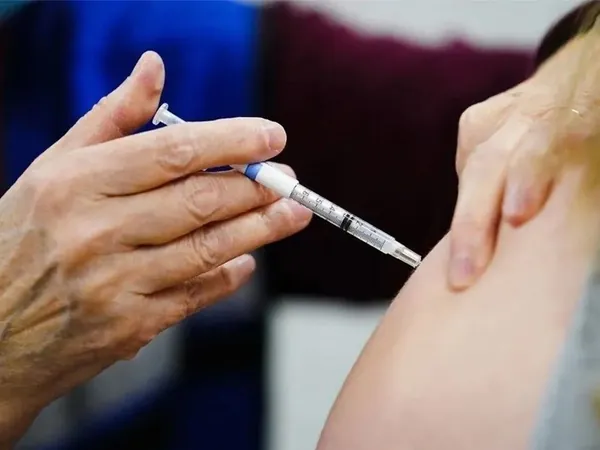
Mater Research in Brisbane, Australia, has launched a pivotal clinical trial for a new combined vaccine targeting both Covid-19 and influenza. This innovative vaccine, developed by Novavax, is particularly aimed at individuals who are unable or unwilling to receive mRNA vaccines like Pfizer or Moderna.
What makes this vaccine unique is its protein-based formulation, which includes a portion of the coronavirus spike protein. By introducing a safe fragment of this spike protein to the body, the vaccine stimulates the immune system to recognize and respond to the virus effectively. When immune cells encounter this protein, they perceive it as a foreign invader and begin preparing a defense, potentially leading to longer-lasting immunity.
The trial is calling for healthy volunteers aged 65 and older who haven’t received an influenza vaccine in the previous two months. This important study is set to kick off on November 4 and will be conducted over a three-week period. Mater Research aims to enroll 150 participants as confirmed by their Respiratory, Infectious Disease, and Thoracic Oncology (RIO) Unit, which is currently overseeing more than 20 clinical trials focused on respiratory health.
Leading the charge in this significant endeavor is Professor Paul Griffin, the Director of Mater Infectious Diseases. His insights underscore the urgency of the pilot: “We know that the majority of people are currently under-vaccinated against both influenza and Covid-19. We hope that offering an approved combination vaccine will drive vaccine uptake, eliminating the need for multiple injections.”
The urgency of this trial is magnified by concerning vaccination statistics: only 559,000 out of 5.56 million Queenslanders have received a Covid vaccine in the past year. Influenza vaccination rates have also dipped, with only 1.7 million doses administered this year compared to last year’s free campaign.
Professor Griffin further elaborated, “Many individuals have concerns about or outright refuse mRNA vaccines. By participating in this trial, people will not only gain access to a non-mRNA vaccine option, but their participation could pave the way for broader approval of this type of vaccine in the future.”
As we witness the ongoing battle against respiratory viruses, this clinical trial represents a significant step towards offering more inclusive vaccination options. Will this combined vaccine be the key to improving public health? Only time will tell. Stay tuned for more updates on this groundbreaking initiative!







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)