Groundbreaking Discovery: Netrin1 Protein Revealed as Key Player in Spinal Cord Development
2024-11-18
Author: Emma
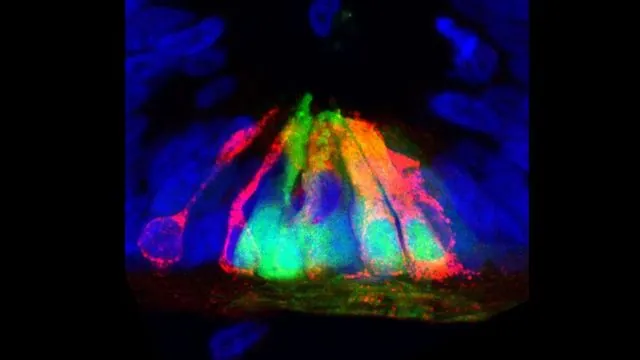
In a groundbreaking study published in Cell Reports, researchers from the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA have unveiled a surprising and critical function of the protein netrin1 in the development of the spinal cord. Long recognized for its role as a guidance cue for nerve fibers, netrin1 also acts as a gatekeeper, ensuring that bone morphogenetic protein (BMP) signaling remains confined to crucial regions in the spinal cord during embryonic development.
The study, led by neurobiology professor Samantha Butler, highlights the importance of netrin1 in fine-tuning the development of the dorsal spinal cord—the area responsible for processing sensory inputs like touch and pain. For the proper formation of sensory neurons, BMP signaling must be meticulously controlled within the dorsal region, preventing it from influencing other areas where different neuron types are formed.
"The regional specificity of signaling molecules like BMP and netrin1 is extremely important for proper neural network formation and function," asserts Sandy Alvarez, the study's first author. "Without netrin1’s regulation, we would likely see a disorganized neural network, which could significantly impact nerve function."
What makes this finding even more remarkable is how it reshapes our understanding of a long-standing concept in neuroscience. In 2017, Butler's team previously challenged the belief that axons were guided by distant cues like netrin1. Instead, they found that netrin1 functions more like an adhesive, affecting axon growth in a more direct manner.
During their recent experiments with chicken and mouse embryos, the researchers discovered an unanticipated side effect: the complete disappearance of specific axon populations when netrin1 levels were manipulated. Their investigation revealed that netrin1 represses BMP activity, thereby altering the patterning of nerve cells in the dorsal spinal cord. In essence, by setting boundaries for BMP signaling, netrin1 ensures that sensory neurons develop where they are needed most.
Furthermore, utilizing advanced techniques in bioinformatics, the team also identified that netrin1 controls RNA translation to regulate BMP activity, further illuminating this complex interaction.
Butler expresses hope for the future: "Our next endeavor will be to understand how we can utilize netrin1 to rebuild circuitry in patients with nerve damage or spinal cord injuries."
The implications of this research extend beyond spinal cord development. Netrin1 and BMP are also found in various other organs, which raises questions about their roles in other developmental processes and diseases. As Alvarez notes, "Our results suggest a need to re-evaluate how netrin1 and BMP interact in other systems, which could enhance our understanding of certain cancers or developmental disorders linked to these proteins."
As scientists continue to unravel the complex web of interactions in neural development, this work lays the foundation for potential therapeutic strategies aimed at aiding neural repair and combating developmental disorders. Stay tuned, as this exciting field of research unfolds further with significant implications for regenerative medicine.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)