Groundbreaking Discovery Offers New Hope for Targeting Cancer-Linked Protein Aurora-A
2024-12-02
Author: Emma
Introduction
In a remarkable breakthrough for cancer research, scientists have unveiled new insights into Aurora-A, a protein that plays a crucial role in cell division and often malfunctions in various cancers. This advances the long-sought quest for effective treatments that target this protein without causing detrimental side effects.
Importance of Aurora-A
Aurora-A belongs to a family of enzymes known as kinases, which are essential for regulating numerous cellular functions. These kinases frequently exhibit irregularities in cancerous cells, creating an opportunity for drug development. However, despite extensive clinical trials aimed at inhibiting Aurora-A, these drugs have yet to make it to clinical application due to the protein's fundamental role in all cell types, which can lead to a host of unwanted toxic effects.
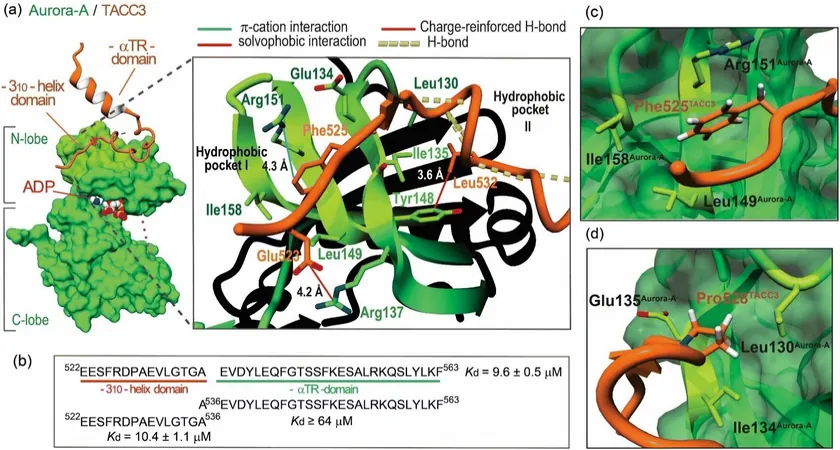
Research Breakthrough
To overcome this critical hurdle, researchers at the University of Birmingham's School of Chemistry have turned their attention to the interactions that Aurora-A has with other proteins, a strategy that may yield more effective anti-cancer therapies. Their innovative findings were recently published in the esteemed journal Chemical Science.
Research Insights from Professor Andrew Wilson
Lead researcher Professor Andrew Wilson states, "Aurora-A is an incomplete kinase, meaning it depends on interactions with other proteins to dictate its activity and location within the cell. By honing in on these interactions, we can potentially achieve a more precise inhibition of Aurora-A."
Peptidomimetics Development
The research team developed a novel strategy using peptidomimetics—a type of synthetic peptide designed to mimic the structure of natural proteins—to specifically disrupt the interaction between Aurora-A and another protein known as TACC3. Remarkably, their most successful peptidomimetics demonstrated the ability to bind with Aurora-A and obstruct its association with TACC3 without inhibiting its essential enzymatic functions.
Implications of Findings
Moreover, the research unveiled an exciting twist: the peptidomimetics not only affected the TACC3 interaction but also altered the conformation of Aurora-A in a way that inhibited another critical protein-protein interaction involving the N-Myc protein. This interaction is vital since aberrations in Myc proteins are notorious markers in cancer progression, especially in aggressive childhood brain cancers.
Future Directions
"By stabilizing N-Myc, Aurora-A contributes to its persistence within the cell. Therefore, disrupting this interaction could lead to promising new strategies for anticancer drug development. Our discovery that peptidomimetics can selectively inhibit the N-Myc/Aurora-A interaction, all while preserving Aurora-A's enzymatic activity, represents a significant advancement in the field," explained Professor Wilson.
Conclusion
As researchers continue to explore this innovative approach, the potential to design more selective and less toxic cancer therapies is brighter than ever. Stay tuned, as this research may illuminate new pathways not only for treating cancer but also for unlocking the broader mysteries of cell division!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)