Groundbreaking Imaging Technique Unveils Ozone-Damaging Molecule’s Reaction to UV Light in Record Time!
2024-11-08
Author: Liam
Introduction
Researchers have achieved a remarkable breakthrough by capturing the astonishing rearrangement of bromoform, a molecule known for its detrimental effects on the ozone layer, within a staggering timeframe of less than a trillionth of a second following exposure to ultraviolet (UV) light. This groundbreaking imaging technique has elucidated a long-suspected pathway through which bromoform interacts with light, significantly contributing to our understanding of its harmful environmental impact.
The Importance of Understanding Chemical Reactions
The sun’s UV rays initiate a myriad of chemical reactions on Earth, many of which occur on incredibly short timescales. Grasping the dynamics of these reactions at an atomic level is crucial not only for comprehending their implications but also for devising strategies to mitigate their damage.
Insights from Dr. Oliver Gessner
Dr. Oliver Gessner, a senior scientist at the Lawrence Berkeley National Laboratory, emphasized the significance of bromoform as a model system for studying these complex interactions. "How do the electrons and atoms interact to facilitate specific chemical reactions? Bromoform plays a vital role in answering this question," he stated.
Bromoform and Its Environmental Impact
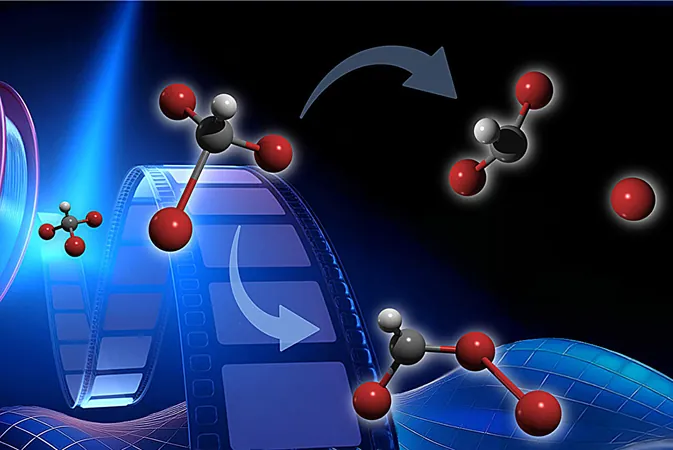
This natural compound, which is primarily produced by oceanic phytoplankton and seaweeds, has long been under scrutiny by chemists worldwide due to its propensity to degrade ozone in the atmosphere. Scientific theories suggest that under UV exposure, bromoform undergoes two distinct processes: dissociation, where a bromine atom detaches from the molecule, and isomerization, where the atoms alter their configuration.
New Findings and Methodology
Despite prior attempts to observe these isomer formation signatures, they were inconclusive due to the transient nature of the phenomenon. The new study published in the *Journal of the American Chemical Society* not only confirms the existence of the isomer but also quantifies the proportions of bromoform undergoing dissociation versus isomerization.
In a pioneering experiment, Gessner and his team initially excited bromoform gas molecules using an ultrafast burst of UV light at a wavelength of 267 nanometers. They then employed ultrashort electron pulses from the SLAC National Accelerator Laboratory's relativistic ultrafast electron diffraction instrument to image these excited molecules. The precision of these measurements was crucial, as the molecules had mere hundreds of femtoseconds to "decide" their reaction pathways.
Results of the Experiment
The analysis from the electron imaging revealed a remarkable insight: approximately 60% of the bromoform molecules proceeded to isomerization in the first 200 femtoseconds of excitation, maintaining this configuration throughout the 1.1-picosecond observation period. Conversely, the other 40% experienced immediate dissociation.
Conclusion and Future Implications
Gessner expressed his excitement about the findings, noting, "It was thrilling to finally visualize the configuration of the isomer that many had predicted." This vital research not only enhances our understanding of bromoform photochemistry but also sheds light on the broader category of UV-induced chemical processes.
The establishment of a benchmark for the isomer formation rate provides a definitive basis for refining theoretical models that predict these types of reactions and their resultant products. The success of this ultrafast imaging technique represents a powerful instrument for addressing urgent environmental questions related to ozone depletion and paves the way for further explorations in photochemical dynamics.
As we continue to grapple with the implications of environmental degradation, studies like this highlight the critical importance of comprehending the underlying processes that drive these chemical reactions, ultimately guiding us towards effective mitigation strategies.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)