Groundbreaking Ion-Channel Research Sheds Light on Cys-loop Receptor Functionality!
2024-12-09
Author: Charlotte
Introduction
In a remarkable breakthrough, researchers from the University of Illinois have unveiled a critical factor that facilitates signal transduction in Cys-loop receptors, a discovery that not only addresses a longstanding mystery in ion-channel physiology but also holds promise for rational drug design. The significant findings are detailed in a recent publication in Science Advances.
Understanding Cys-loop Receptors
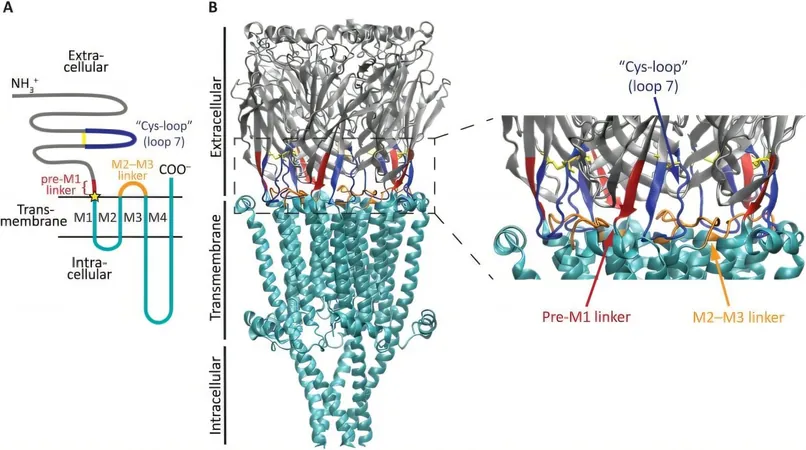
Cys-loop receptors are a pivotal class of ligand-gated ion channels known for their two major domains connected by a short linker. Positioned in the cell membrane, the extracellular top domain responds to external signals, like neurotransmitters, while the bottom domain allows ions to traverse the membrane upon activation.
A Historical Mystery
Historically, the intricate communication between the top and bottom domains has puzzled scientists. As external molecules bind to the top domain, how does the bottom domain become aware of this binding? Researchers from Claudio Grosman’s lab have made significant strides in answering this question, employing a unique approach that deviates from traditional studies focused solely on the interfacial region—the area intersecting the two domains.
Key Discoveries
Utilizing loss-of-function mutations, the team discovered that the distance between the two domains is crucial for successful signal transduction. Contrary to previous beliefs suggesting that communication could be disrupted through substitutions in the interfacial region, their findings revealed that signals could still convey even with extensive mutations in this area.
Postdoctoral researcher Nicole Godellas highlighted their innovations, stating, 'When a channel is electrically silent, we know that it is present in the cell, but it fails to allow ions to pass through.' This condition arises when mutations effectively hinder the communication needed for the top domain's ligand binding to influence the bottom domain.
Linker Mutations and Communication
Through experiments involving linker mutations, Godellas and her colleagues observed a critical separation between the two domains. Similar to a magnetic interaction being disrupted by an intervening object, the insertion of additional amino acids acted as a buffer, impairing the communication pathway essential for ion flow.
Implications of the Findings
Moreover, the research team employed a competition ligand-binding assay to probe canonical binding sites, leading to the revelation that the linker region exhibits a narrow tolerance for amino acid insertions or deletions. This provides evolutionary insights into why this specific length is preserved in Cys-loop receptors.
Future Directions
Moving forward, the team aims to explore the structural implications of linker mutations, potentially offering answers to what restricts signal transduction in these vital channels. This foundational research carries immense significance, particularly given the therapeutic applications of Cys-loop receptors in treating inflammatory, neurological, motor, and psychiatric disorders.
Conclusion
As Godellas affirms, 'We are delving into the mechanistic and molecular operations of these membrane receptors, laying the foundation for medicinal chemists to create targeted, safer, and more effective drugs. Our basic science is paving the way for rational drug design.' This groundbreaking study not only enhances our understanding of ion-channel physiology but may also expedite advancements in drug development, pushing the boundaries of therapeutic interventions in various health conditions.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)