Groundbreaking Study Unveils Secrets to Early Embryonic Development in Drosophila
2024-11-18
Author: Charlotte
Introduction
Researchers have made a significant leap in understanding how early embryos transition from maternal support to their own genetic control through a process known as zygotic genome activation (ZGA). A recent study led by Chinese scientists has shed light on the complex molecular mechanisms that orchestrate this critical transition in early Drosophila embryos.
Study Overview
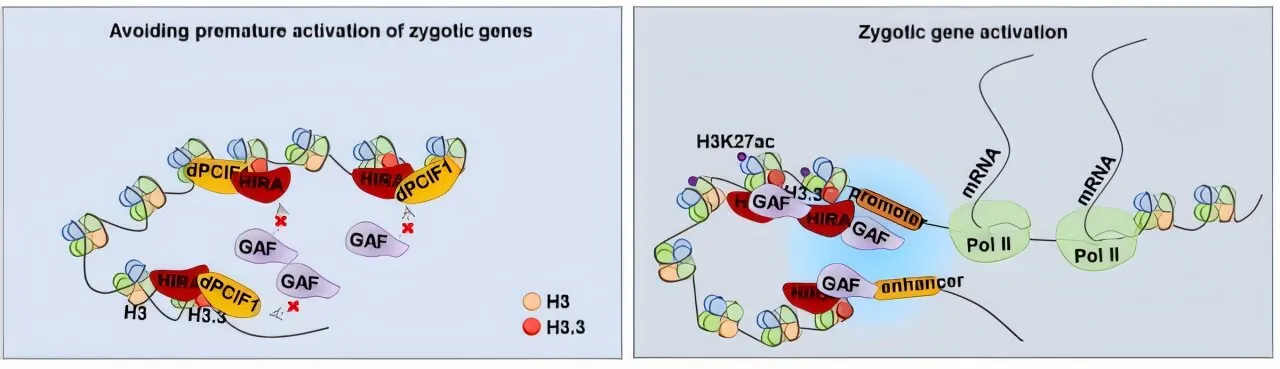
Published on November 14 in the Proceedings of the National Academy of Sciences, the study is titled “HIRA and dPCIF1 coordinately establish totipotent chromatin and control orderly ZGA in Drosophila embryos.” This research reveals how the totipotent state of chromatin is generated, permitting the embryo to activate its own genes in a well-coordinated manner.
Key Findings
In the earliest stages of development, embryos rely entirely on maternal RNAs and proteins, remaining transcriptionally silent as their own genome lies dormant. However, as development progresses, the embryo undergoes profound genomic reorganization, transforming the chromatin from a dormant germ cell state to an active totipotent state. This transformation is crucial for successful development as the embryo begins to express its own zygotic genes.
Role of Transcription Factors
Key players in this intricate process are the pioneer transcription factors Zelda and GAF. These factors work together during the maternal-to-zygotic transition to activate zygotic genes, ensuring that the embryo transitions smoothly through various developmental stages.
Research Team and Methodology
Professor Sun Qinmiao and his team from the Institute of Zoology at the Chinese Academy of Sciences, in collaboration with Chen Dahua's team from the Biomedical Research Institute of Yunnan University, focused on deciphering how these transcription factors are regulated, particularly on the establishment and maintenance of totipotent chromatin in these early stages.
Impact of dPCIF1 and HIRA
The researchers discovered that dPCIF1, a regulatory factor, is vital for this process. When maternal dPCIF1 is depleted, embryos showed a marked increase in embryonic lethality alongside heightened binding of the pioneer transcription factor GAF to early embryonic chromatin. This suggests that dPCIF1 serves as a regulatory checkpoint, mitigating GAF's activity to prevent premature gene activation.
Conclusion
Additionally, the study identified HIRA, a histone chaperone that interacts with both dPCIF1 and GAF. The absence of HIRA also resulted in total embryonic lethality and a substantial decrease in zygotic gene expression. Notably, the zygotic genes whose expression was downregulated in the absence of HIRA were found to be those that were prematurely activated in embryos lacking dPCIF1.
As Prof. Sun articulates, “dPCIF1 acts as a surveillance factor, assisting HIRA in achieving orderly zygotic genome activation by controlling the premature activation of GAF.” This insight not only enhances our understanding of embryonic development in Drosophila but may also have broader implications for developmental biology and reproductive health in other species.
As researchers continue to unravel the complexities of embryonic development, this study opens new avenues for exploration in the regulation of gene expression during critical early life stages. The findings highlight the delicate balance of factors necessary for proper embryogenesis and raise intriguing questions about the potential for targeted interventions in the event of developmental anomalies.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)