Revolutionary Bioluminescent Immunosensor Set to Transform Point-of-Care Testing!
2024-12-17
Author: Charlotte
A groundbreaking nanobody-based immunosensor has emerged as a game-changer for point-of-care testing (POCT), showing extraordinary promise in various fields. Developed by researchers at Science Tokyo, this innovative device harnesses the power of bioluminescence resonance energy transfer (BRET), demonstrating potential applications in therapeutic drug monitoring and environmental assessments, all while functioning effectively in undiluted biological fluids.
Immunosensors have become essential in biochemistry and medical science, allowing for precise detection of specific biomolecules by utilizing the interactions between antibodies and their corresponding target antigens. Their relevance spans across clinical diagnostics, food safety monitoring, and environmental surveillance.
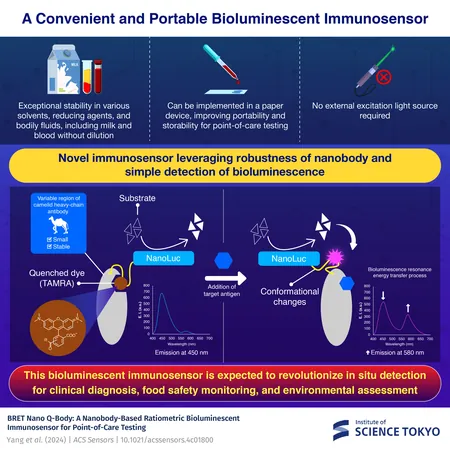
As the demand increases for efficient and cost-effective testing solutions, scientists are continually pushing the boundaries of immunosensor technology. A notable trend is the development of homogeneous immunosensors that do away with the need for labor-intensive washing steps. Enter the Quenchbodies (Q-bodies), a promising class of homogeneous immunosensors that utilize fluorescence to signal antigen binding. While Q-bodies are impressive, they fall short in real-world applications, especially within undiluted biological fluids like blood and milk.
Recognizing these limitations, an innovative team led by Associate Professor Tetsuya Kitaguchi at the Institute of Science Tokyo has successfully engineered a new variant of Q-bodies that circumvents these issues. Their results, published on November 11, 2024, in *ACS Sensors*, could revolutionize clinical and environmental immunosensor applications.
This new immunosensor combines the advantages of robust nanobodies—small, highly stable antibody fragments from camels—with NanoLuc, a luciferase enzyme that emits blue light upon reaction with its substrate. The combination of these elements allows the sensor to function optimally in undiluted biological fluids, enhancing its application range beyond conventional limitations.
The operational mechanism is ingenious: upon the binding of a target antigen to the nanobody, significant structural changes transpire, favoring a movement of the fluorescent dye, TAMRA, towards the NanoLuc enzyme. This proximity enables an energy transfer that alters the emission from blue to red, thereby allowing for precise quantification of the antigen based on the emission intensity ratio.
The research team conducted extensive experiments to validate this technology. “Our BRET nano Q-body boasts exceptional thermostability and is resilient against various environmental stresses, making it suitable for direct application in biological samples—even in complex matrices like serum and whole blood,” Kitaguchi revealed.
Going further, the team tested the implementation of these BRET nano Q-bodies in paper-based devices designed to measure chemotherapeutic drug concentrations. Impressively, these devices maintained their efficacy post long-term storage without temperature controls, proving their worth in practical scenarios—be it bedside testing, field analyses, or home healthcare.
As a testament to its potential, the researchers foresee these paper-based immunosensors revolutionizing in situ detection in therapeutic, diagnostic, and environmental contexts. This pioneering study exemplifies how the integration of diverse analytical biochemistry tools can culminate in transformative technology that promises to enhance healthcare and environmental stewardship.
Stay tuned, as this might just be the future of accessible and reliable diagnostics at your fingertips!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)