Revolutionary Gel for Bone and Tissue Regeneration Shows Promise in Rat Studies
2024-11-18
Author: William
Introduction
In an exciting breakthrough, researchers from Queen Mary University of London and the University of Nottingham have unveiled a groundbreaking biocooperative material designed to harness the body’s natural healing processes. This innovative gel uses blood clotting and peptide self-assembly techniques to create personalized regenerative implants capable of healing severe wounds and fractures.
Challenges in Regenerative Medicine
As medical science advances, the opportunity for effective regenerative therapies for treating complex injuries is becoming more tangible. However, replicating the intricate environment required for the body’s natural healing remains a formidable challenge. Current approaches often utilize stem cells, biomimetic materials, or allogeneic grafts, each presenting its own hurdles to successful application in clinical settings.
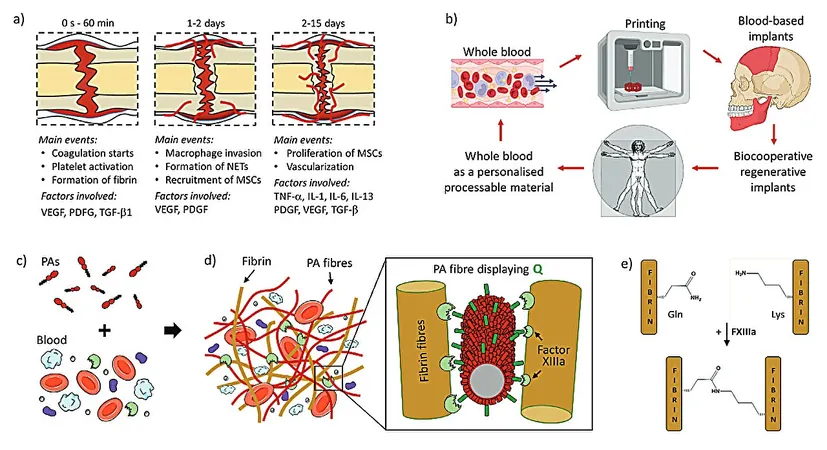
The Mechanism of Healing
Most tissues in the human body are adept at recovering from minor injuries, primarily by forming a regenerative hematoma that orchestrates molecular and cellular actions necessary for complete healing. The new study, titled "Biocooperative Regenerative Materials by Harnessing Blood-Clotting and Peptide Self-Assembly," published in *Advanced Materials*, sheds light on how utilizing the body’s own components can enhance healing.
Innovative Gel Development
The researchers engineered peptide amphiphiles (PAs) to engage with blood during the coagulation process, forming a living material that closely resembles a regenerative hematoma. By cleverly co-assembling these PAs with a patient's own blood components, the team created hydrogels that exhibit crucial properties needed for healing.
Optimized Interactions
Designed with varying charge densities, the PAs were optimized to interact with essential proteins like fibrinogen and albumin. They even incorporated glutamine residues to facilitate the action of the enzyme Factor XIIIa, which strengthens the gels by linking them with fibrin, thereby augmenting their mechanical attributes.
Mechanical Performance
Mechanical tests have shown that the gel's stiffness can be finely tuned, and its structure boasts a robust network that mimics natural blood clots. Scanning electron microscopy revealed a remarkable nanofibrous architecture with platelets behaving as expected, spreading out to aid in healing.
Growth Factors and Cell Support
Moreover, these materials not only support normal platelet function but also generate a continuous supply of growth factors, fostering the growth of crucial cell types like mesenchymal stromal cells, endothelial cells, and fibroblasts in laboratory settings.
3D Printing Compatibility
An impressive highlight of this research is the gel’s compatibility with 3D printing technology, enabling the on-site fabrication of personalized implants with precise geometries needed for specific injuries.
In Vivo Results
In in vivo trials utilizing a rat skull defect model, the results were promising. The PA-blood gel implants achieved 62% and 56% new bone formation, significantly outperforming commercially available Bio-Oss (50%) and untreated defects (30%). This remarkable efficacy underscores the potential for this material to revolutionize clinical therapeutic applications.
Future Implications
By tapping into the sophisticated strategies honed by billions of years of evolution, this biocooperative approach marks a significant step toward accessible and individualized regenerative therapies. While there are still obstacles to overcome before this technology can be translated into human medicine, the study signifies a hopeful leap forward in the field of regenerative healing.
Conclusion
As researchers continue their work, the medical community eagerly awaits developments that could make this revolutionary treatment available for those suffering from severe injuries in the near future!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)