Revolutionary Hybrid Catalyst Boosts Clean Oxygen Production – A Game Changer in Sustainable Energy!
2024-11-29
Author: Olivia
In a groundbreaking development for sustainable energy, a research team from the Institute of Materials Chemistry at TU Wien, headed by Professor Dominik Eder, has unveiled a new synthetic method to create robust, conductive, and catalytically active hybrid framework materials specifically designed for (photo)electrocatalytic water splitting. This study has been highlighted in the prestigious journal, Nature Communications.
Producing sustainable energy carriers like hydrogen (H2) is crucial in our battle against climate change, and one of the most promising methods is splitting water into hydrogen and oxygen (O2). This can be achieved either through electrochemical methods or light-assisted processes, or a combination of both. However, a highly efficient catalyst that accelerates this reaction without being depleted is essential for this process. The catalyst must feature a large surface area to facilitate the adsorption and splitting of water molecules, as well as exceptional durability for extended use.
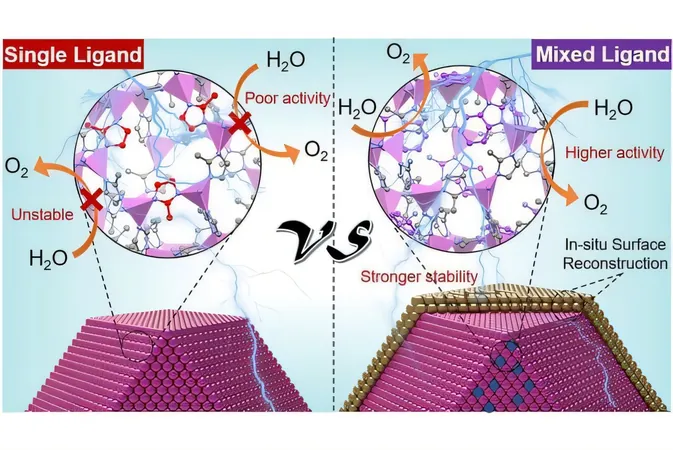
Enter zeolitic imidazolate frameworks (ZIFs): These hybrid organic/inorganic materials boast impressive surface areas and plentiful adsorption sites, making them ideal for catalytic applications. ZIFs are composed of metal ions, such as cobalt, intricately linked by organic molecules known as ligands through coordination bonds, yet conventional ZIFs typically only utilize a single type of ligand.
Professor Eder notes a significant limitation: “These ZIFs often lack stability in water under electrocatalytic conditions, which is crucial for long-term application. Their relatively low electronic conductivity also hinders their performance in electrocatalytic scenarios.”
To counter these limitations, the research team has pioneered a method to engineer ZIFs with two or more organic ligands. “We needed to strategically combine both ligands to achieve a uniform mixture throughout the framework while preserving the ZIF's original structure,” explains lead author Zheao Huang. The team meticulously evaluated various combinations of ligands and process parameters, ultimately discovering the most effective pairing.
Unlocking Synergistic Advantages of Dual Ligands
The research unveiled that this innovative modification greatly enhances ZIF stability, prolonging durability in electrocatalytic water splitting from mere minutes to a remarkable one-day lifespan. Utilizing advanced spectroscopic and microscopic techniques alongside computational theories in partnership with Central China Normal University, the team identified how the ideal ligand combination significantly bolstered the coordination bond with cobalt ions. This synergy prevented the porous structure from collapsing during experimental tests.
"Remarkably, after just a few minutes into the reaction, we detected the formation of an ultra-thin film of cobalt oxyhydroxide—just a few nanometers thick—on the ZIF nanoparticles, which safeguarded the material from degradation and structural collapse," Huang elaborates.
Moreover, the dual ligand approach increased the conductivity of the ZIF material by an astonishing factor of ten, which subsequently accelerated the oxygen evolution reaction (OER) rate by the same magnitude.
Professor Eder remarked on the findings, saying, “Our simulations indicated that the interaction between the two ligands creates a high density of mobile charge carriers throughout the material. While we anticipated some improvements, we were genuinely astonished by how dramatically our new strategy enhanced the (photo)electrocatalytic performance of ZIFs.”
This revolutionary hybrid catalyst not only pushes the boundaries of current water-splitting technologies but also stands as a beacon of hope in the pursuit of environmentally friendly energy solutions. As research continues, the implications of this advancement could be monumental in transforming the future of sustainable energy production. Could this be the key to unlocking efficient hydrogen production and combating climate change? Only time will tell!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)