Revolutionary New Enzyme Created by Scientists: The Future of Greenhouse Gas Utilization?
2024-12-02
Author: Sophie
Revolutionary New Enzyme Created by Scientists: The Future of Greenhouse Gas Utilization?
In a groundbreaking study led by Tobias Erb at the Max Planck Institute for Terrestrial Microbiology in Marburg, researchers have successfully engineered a novel enzyme named “lactyl-CoA mutase” that greatly enhances the ability to convert a fundamental metabolic compound into valuable chemical products. This innovative development could hold key implications for the sustainable capture and utilization of the greenhouse gas CO2.
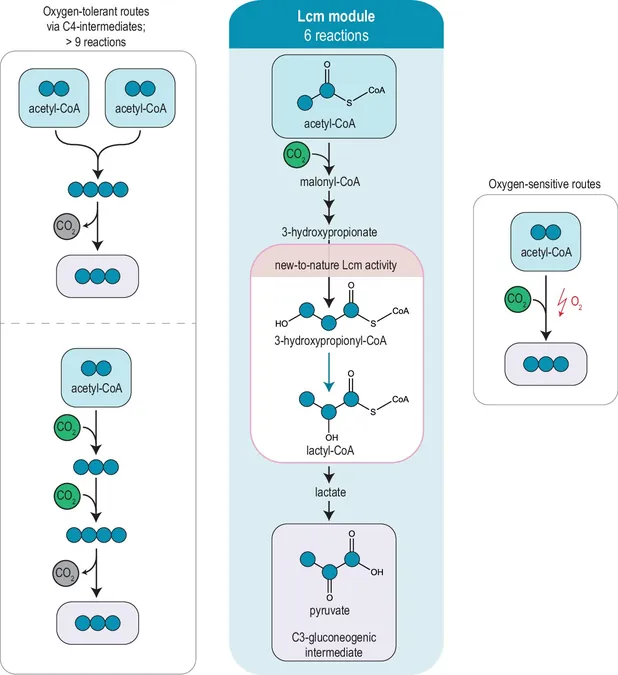
The research, which can be found in the prestigious journal Nature Communications, highlights the importance of acetyl-coenzyme A (acetyl-CoA) as a critical building block in cellular metabolism. As a central player in various CO2 fixation pathways, acetyl-CoA's role is vital: its effective conversion within the cell dictates biomass formation and ultimately influences how efficiently CO2 can be utilized in biotechnological applications.
Traditionally, converting acetyl-CoA into simpler cell intermediates, such as pyruvate, has been fraught with inefficiencies. These natural metabolic pathways often involve multiple steps or require specific conditions, leading to the loss of valuable carbon. Recognizing these limitations, Erb's team set out to construct a direct and efficient metabolic “bridge” that would streamline the transition from acetyl-CoA to pyruvate, aiming simultaneously to harness CO2 in the process.
Innovation Through Theoretical Design
In the realm of synthetic biology, innovative metabolic pathways are often conceptualized on paper before undergo laboratory testing. For this project, the researchers outlined an optimal pathway designed to incorporate additional CO2 while remaining shorter than previously established routes. However, the enzyme necessary for this transformation, lactyl-CoA mutase, was merely a theoretical concept—its activity had not been documented in the natural world.
The research team struck gold when they identified an enzyme candidate from existing databases that held promise for their desired reaction. While initial tests confirmed that this candidate could process the requisite substrate, it did so at a painfully sluggish rate.
Accelerating Nature’s Processes
Recognizing that evolution drives improvement in enzyme efficiency, the researchers, led by doctoral student Helena Schulz-Mirbach, devised a lab-based accelerated evolution strategy. By coupling the enzyme’s activity to the growth of a modified Escherichia coli strain, they were able to promote and select for faster enzyme variants. This innovative approach resulted in lactyl-CoA mutase variants that exhibited a remarkable 5- to 10-fold increase in efficiency in laboratory tests, creating opportunities for practical applications.
Philipp Wichmann, another key author of the study, emphasized the integration of metabolic and evolutionary mechanisms to refine enzyme properties, showcasing the potential for synthetic biology and cell-free biochemistry breakthroughs.
The Road Ahead: Enhancements and Applications
Despite these advancements, the research team acknowledges the need for further improvements to the lactyl-CoA mutase to increase its speed and application range. Future research aims to not only optimize the enzyme’s activity but also to delve deeper into understanding its structure and reaction mechanisms, a journey that promises even more insights into biological processes.
This fresh metabolic pathway holds immense potential beyond CO2 utilization; one promising application could be in the sustainable production of 3-hydroxypropionate, a key precursor for environmentally friendly, biologically derived plastics.
As the study continues to unfold and more is revealed regarding the capabilities of lactyl-CoA mutase, the scientific community may very well be on the cusp of a new era in green biotechnology, potentially transforming the way we manage CO2 emissions and paving the way for a more sustainable future. Stay tuned for further updates as these researchers push the boundaries of what synthetic biology can achieve!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)