Revolutionary Water-Repelling Material Set to Transform Everyday Surfaces!
2024-12-12
Author: Olivia
Introduction
Scientists from the Karlsruhe Institute of Technology (KIT) and the Indian Institute of Technology Guwahati (IITG) have made a groundbreaking advancement by creating a new surface material that exhibits almost complete water repellency. Through an innovative approach, they modified metal-organic frameworks (MOFs)—next-generation materials known for their unique properties—by attaching hydrocarbon chains to them.
Potential Applications
These newly engineered superhydrophobic surfaces hold great potential for applications in self-cleaning surfaces across various sectors, including automotive and architectural designs, where resistance to environmental elements is paramount. The findings of this significant study were recently published in the prestigious journal *Materials Horizons*.
Understanding MOFs
MOFs, made from metals and organic linkers, form a porous network reminiscent of a sponge, creating a vast surface area. To illustrate, just two grams of this material can cover the area of a football pitch! As a result, these frameworks are not just innovative in water repellent technology, but they are also highly valuable in fields like gas storage, carbon dioxide capture, and even cutting-edge medical technology.
Research Breakthroughs
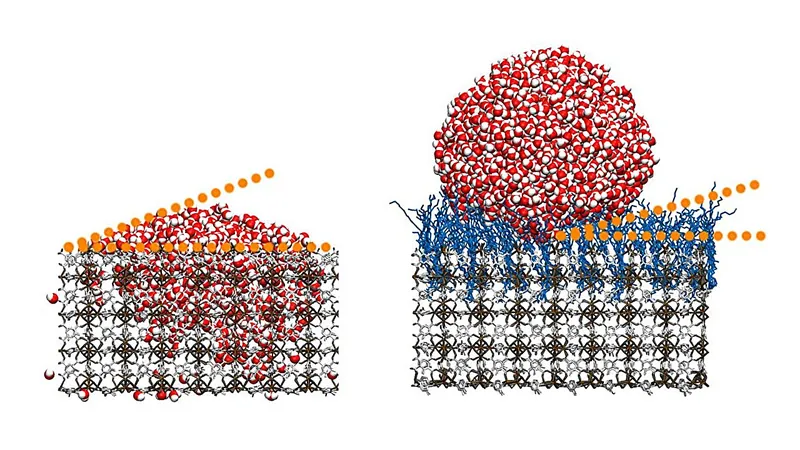
The research team achieved noteworthy results by grafting hydrocarbon chains onto thin films of MOF, reaching an impressive water contact angle greater than 160 degrees. According to Professor Christof Wöll from KIT, “Our technique allows us to create superhydrophobic surfaces with contact angles much higher than those found in other common coatings.” This achievement marks a pioneering use of monolithic MOF thin films for hydrophobic applications.
A Game Changer in Material Science
The team believes the exceptional water-repelling properties result from the unique arrangement of the hydrocarbon chains on the MOFs. When grafted, these chains form a disordered state known as a “high-entropy state,” essential for maximizing hydrophobic performance. In a surprising twist, the researchers discovered that even when using perfluorinated hydrocarbon chains—which typically enhance water repellency in materials like Teflon—the water contact angle decreased significantly, showcasing the unique advantages of their hydrocarbon modifications.
This discovery was backed by computer simulations that revealed perfluorinated molecules failed to achieve the energetically favorable high-entropy state, unlike their hydrocarbon counterparts. The team also experimented with manipulating the surface roughness of their systems at the nanoscale, further enhancing the material’s hydrophobicity and self-cleaning capabilities.
Professor Uttam Manna from IITG emphasized the importance of their theoretical analysis, stating, “Our detailed study links unexpected experimental behaviors to the high-entropy state of molecules, paving the way for the next generation of materials designed with optimal hydrophobic properties.”
Conclusion
With such groundbreaking developments, the future of self-cleaning and water-resistant technologies appears brighter than ever. As the demand for high-performance materials grows, this innovation is set to revolutionize how we interact with our environments—making tedious cleaning tasks a thing of the past! Stay tuned for further advancements in material science that promise to change our world!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)