Revolutionizing Drug Development: New Strategy for Nitrogen Insertion Unveiled!
2025-01-06
Author: William
Introduction
In the world of pharmaceuticals, even the smallest changes often lead to monumental shifts in drug efficacy and properties. A key player in this arena? Nitrogen. Research shows that a staggering 80% of small-molecule drugs approved by the US Food and Drug Administration from 2013 to 2023 contain a nitrogen atom in their structure. Now, a breakthrough in skeletal editing is set to provide chemists with innovative tools to modify these crucial nitrogen atoms in a precise and efficient manner.
The Breakthrough Strategy
Researchers at the University of Oklahoma have developed a groundbreaking skeletal editing strategy that enhances five-membered aromatic rings already containing nitrogen by integrating an additional nitrogen atom. This cutting-edge technique, detailed in a recent publication in *Science*, offers a novel reagent that operates without additives, requires no protection on pre-existing nitrogen atoms, and does not interfere with sensitive oxidized groups, allowing for subtle modifications in drug leads.
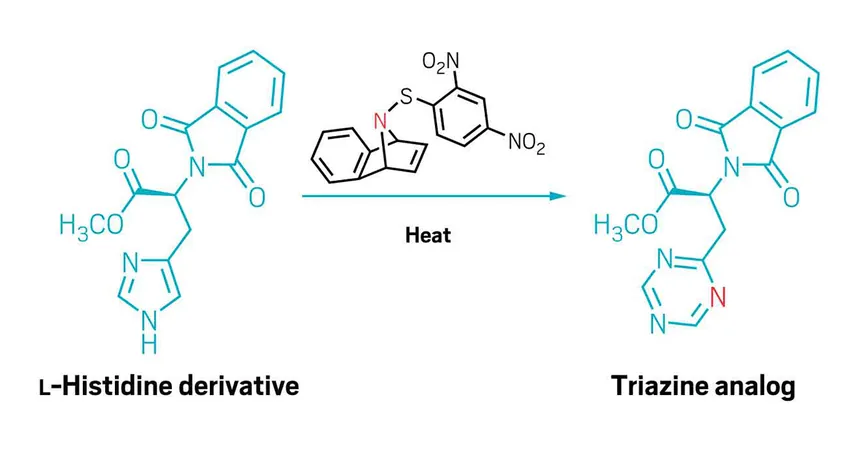
The Role of Sulfenylnitrenes
Lead researcher Indrajeet Sharma emphasizes the need for a versatile method that accommodates all types of functional groups. His team achieved this by utilizing sulfenylnitrenes—reactive nitrogen atoms bound to sulfur—which provide both stability and a leaving group during the reaction process.
Reagent Development
The researchers crafted various bench-stable reagents that, when heated, decompose into these reactive sulfenylnitrenes. Intriguingly, by altering the aromatic ring connected to the sulfur, they can adjust the activation temperature for the nitrene, making it adaptable for different molecules. Sharma noted that they are in the process of making these reagents commercially available, transforming the landscape of drug modification.
Reaction Mechanism
During the reaction, the nitrene attaches to an electron-rich double bond neighboring the existing nitrogen. This leads to the departure of the sulfur group and a structural rearrangement that incorporates the new nitrogen atom. Notably, should two heterocycles be present in a molecule, the added nitrogen selects the more electron-rich ring.
Wide Applicability
The team's findings reveal that their sulfenylnitrene approach effectively introduces nitrogen into nearly any five-membered aromatic N-heterocycle, encompassing compounds such as pyrroles, indoles, azaindoles, and imidazoles—previously challenging candidates for nitrogen insertion. They illustrated the method's compatibility with a range of functional groups by successfully modifying various drug molecules and complex structures, including amino acid derivatives and sensitive thioethers.
Future Directions
Looking ahead, Sharma's team is focused on expanding this method to incorporate nitrogen into rings without any initial nitrogen presence. Additionally, they are investigating ways to enhance reaction yields within safer solvation environments, as the current method shows optimal activity in toxic chlorobenzene, raising concerns in industrial settings.
Expert Opinions
Richmond Sarpong from the University of California, Berkeley, who specializes in skeletal editing methodologies, praised this advancement as a significant enhancement to the existing repertoire of single-atom skeletal editing techniques. He commended the adaptability of the reagents across various temperatures, suggesting that this innovative approach will attract wide interest among chemists.
The Future of Drug Development
Sharma envisions a future where artificial intelligence interplays with skeletal editing techniques to tackle drug development challenges. By pinpointing structural weaknesses in drug candidates, chemists could make strategic modifications rather than starting anew, describing it as a "renovation" approach to pharmaceutical innovation.
Conclusion
This breakthrough not only showcases the potential for advancing drug design but also highlights the synergy between chemistry and technology, promising a new era of efficient and targeted drug development strategies. Stay tuned, as these developments could change the face of pharmaceuticals forever!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)