Revolutionizing Propane Conversion: How Researchers Use Sunlight and Water to Create Propylene
2025-04-15
Author: Emma
A Game-Changer in Catalysis
Researchers have unlocked a groundbreaking method to transform propane into propylene using just a sprinkle of water and the power of sunlight. Traditionally, the propane dehydrogenation (PDH) process demands scorching temperatures over 600°C, leading to hefty energy costs and wear on catalysts. This new approach promises a less energy-intensive and more efficient solution.
Innovative Breakthrough in Ambient Conditions
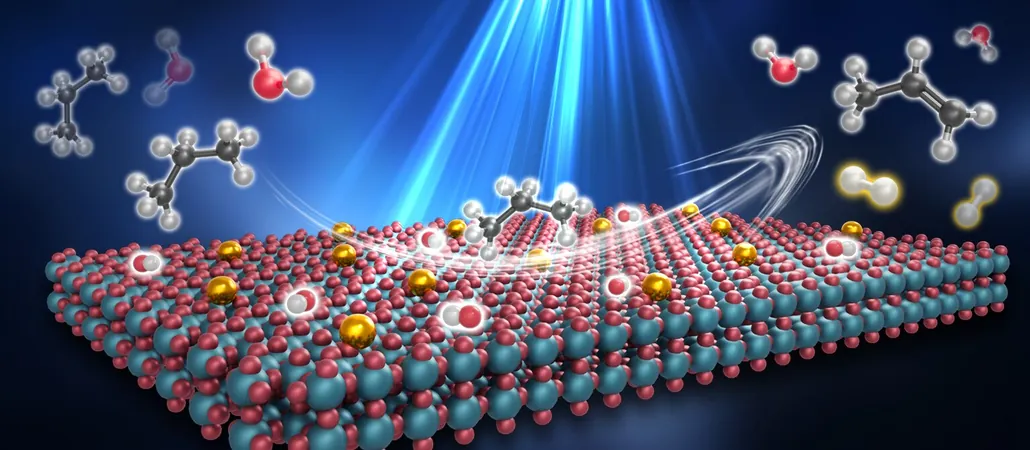
In a cutting-edge study published in Nature Chemistry, a team led by Professors Zhang Tao and Wang Aiqin from the Dalian Institute of Chemical Physics, in collaboration with Professor Gao Yi’s group at Shanghai Advanced Research Institute, has pioneered a water-assisted PDH route by employing a copper single-atom catalyst (SAC). This method allows for the efficient conversion of propane to propylene at an astonishingly low temperature of just 50-80°C.
The Role of Water and Light
Utilizing a Cu1/TiO2 SAC, the researchers engineered a reaction environment where water vapor, copper atoms, and light work synergistically to facilitate the reaction. During the process, photocatalytic water splitting generates hydrogen and hydroxyl species, which then prompt a chemical reaction that converts propane into propylene while producing water, all without depleting the water used.
A Versatile Method for Other Gases
What’s more, this revolutionary process isn’t limited to propane—it can also extend to other light alkanes such as ethane and butane. Remarkably, the catalytic reactions can even be powered by direct sunlight, setting a new standard for sustainable energy practices.
Setting a New Precedent
"This study not only introduces a novel pathway for propane dehydrogenation but also lays the groundwork for high-temperature reactions harnessed by solar energy," remarked Professor Liu Xiaoyan, a leading author of the research. With this significant advancement, the future of energy-efficient chemical processes looks brighter than ever.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)