Shocking Discovery: Genetic Variants Linked to Alzheimer's Cause Brain Inflammation in Women—New Study Reveals!
2024-09-30
Author: Olivia
Groundbreaking Study from Weill Cornell Medicine
In a groundbreaking study from Weill Cornell Medicine, researchers have uncovered alarming evidence that two genetic variants significantly heighten the risk of Alzheimer's disease (AD) and trigger dangerous inflammatory responses in the brains of females. Published on September 30 in Neuron, this research underscores the pressing need to consider sex differences in the quest for effective Alzheimer’s treatments.
Understanding Alzheimer's Disease Risk
Alzheimer’s disease is a pressing global health issue, affecting millions, with women facing almost double the risk of developing the disease compared to men. As scientists work tirelessly to uncover the reasons behind this disparity, the latest findings shed light on the underlying mechanics of these genetic risk factors.
The Role of Genetic Variants
Previous research had already flagged the APOE4 gene variant as a critical risk factor for AD, particularly in women. This new study takes things a step further by spotlighting the interactions between APOE4 and the TREM2 gene, specifically the R47H variant—a rare mutation linked to a 2-4.5-fold increased risk of Alzheimer’s.
Research Methodology and Findings
Dr. Li Gan, the senior author and prominent figure in neurodegenerative disease research, stated, “Despite these variants being among the strongest risk factors for Alzheimer’s, we still lack a comprehensive understanding of how they combine to amplify disease risk.” To address these gaps, Dr. Gan and her dedicated team, including lead researcher Dr. Gillian Carling, designed a preclinical model using mice genetically engineered to express human versions of APOE4 and TREM2 R47H.
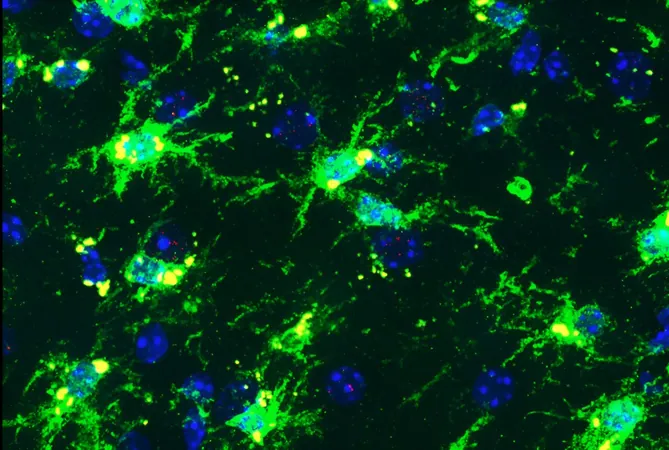
At around 9-10 months old—analogous to middle age in humans—these mice were analyzed for the effects of these genetic variants on their brain health. Results revealed alarming findings: female mice with both genetic risk factors exhibited significant damage in critical brain regions associated with memory and cognition. This damage was characterized by a marked increase in tau protein clumps—hallmarks of Alzheimer’s pathology—compared to their non-gene-combination counterparts.
Microglial Dysfunction and Inflammation
The study points to the role of microglia, the brain’s immune cells, which normally provide protection but appeared to behave dysfunctionally in the presence of these harmful variants. Described as "senescent," these aging microglia failed to effectively clear cell debris and instead released inflammatory chemicals through a pathway called cGAS-STING. Astonishingly, this negative effect was observed only in females.
Significance of cGAS-STING Pathway
Dr. Carling elaborated, “Our findings indicate that when these two Alzheimer’s risk factors converge in females, the cGAS-STING pathway is drastically activated.” The research team noted that inhibiting this damaging pathway significantly reduced harmful inflammatory factors and restored normal function in the microglial cells.
Implications for Alzheimer's Research and Treatment
This pivotal research highlights the necessity of considering biological sex in Alzheimer’s studies and potential treatment approaches. As the disease manifests differently in men and women, a one-size-fits-all treatment may not be effective. Dr. Gan emphasized that understanding immune pathways like cGAS-STING is crucial for recognizing how Alzheimer’s disease progresses, particularly in those with heightened genetic risks.
Future Directions
The implications of this study are profound as they promise new avenues for treatment and prevention. As researchers continue to delve deeper into the interplay between genetics and immune response in Alzheimer’s, there’s hope for breakthroughs that could change the landscape of how we approach this debilitating disease.
Stay tuned as we bring you more updates on this developing story and the future of Alzheimer’s research!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)