The Surprising Link Between Nucleolus Size and Aging: Could It Be the Key to Extending Lifespan?
2024-11-25
Author: Charlotte
In a groundbreaking study, researchers from Weill Cornell Medicine have unveiled that the size of a cell's nucleolus—a vital structure nested within the nucleus—may play a crucial role in determining cellular aging. The research, published on November 25 in Nature Aging, involved yeast as a model organism, highlighting both its simplicity and striking similarities to human cellular processes.
The findings could pave the way for innovative treatments targeting longevity, potentially extending the human lifespan by addressing the root causes of age-related diseases. As the aging process is a major risk factor for debilitating illnesses such as cancer, cardiovascular disease, and neurodegenerative disorders, understanding the mechanisms that contribute to aging is becoming increasingly critical.
Dr. Jessica Tyler, a leading researcher in the study, emphasizes that combating these diseases through a singular therapeutic approach—rather than treating each ailment individually—could transform the way we think about health in older age. "Aging is the highest risk factor for these diseases," she noted, reinforcing the idea that targeting the aging process itself might be the most effective strategy for enhancing health and longevity.
What's Happening Inside the Nucleus?
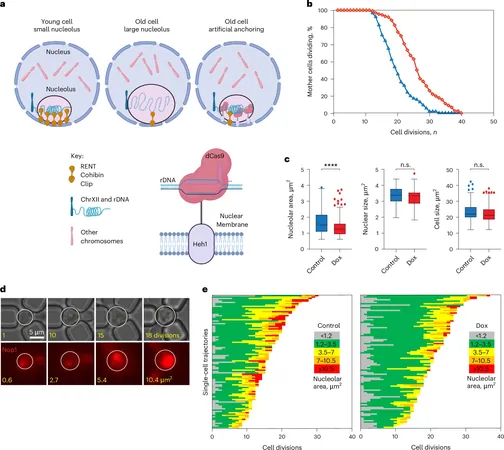
The nucleus is home to the cell's chromosomes, while the nucleolus is crucial for housing ribosomal DNA (rDNA), which is essential for producing ribosomes—cellular machinery responsible for protein synthesis. The rDNA is particularly vulnerable to damage, given its repetitive structure. Any failure to repair this damage can provoke catastrophic cellular consequences, including genomic instability and, ultimately, cell death.
Interestingly, as organisms age—ranging from yeast to worms and humans—the nucleolus tends to expand. This expansion is associated with detrimental effects, while interventions like calorie restriction have been shown to maintain a smaller nucleolus. "Calorie restriction does many different things, and no one knows the precise way that it extends lifespan," Dr. Tyler remarked.
In a pioneering experiment, Dr. Tyler and postdoctoral fellow Dr. J. Ignacio Gutierrez engineered a method to control the anchoring of rDNA to the nuclear membrane of yeast cells. This manipulation allowed them to focus solely on the nucleolus's size without interference from other anti-aging variables.
The results were astonishing. By keeping the nucleolus compact, the researchers observed that it delayed aging to an extent comparable to calorie restriction. This discovery can shift the narrative in aging research, indicating that cellular structure could be as vital as dietary choices in promoting longevity.
The Mortality Timer Revelation
What stood out in the research was the discovery that nucleoli do not expand uniformly throughout a cell's lifespan. Instead, they remain small for the majority of life, only to surge in size dramatically once a threshold is crossed—this event marks a significant turning point for the cell, leading to an average potential of merely five additional cell divisions. This points to a remarkable characteristic of nucleoli serving as a "mortality timer," ticking down the last moments of a cell’s life.
As cells accumulate DNA damage over time, the stability of rDNA also diminishes. The study revealed that larger nucleoli contained less stable rDNA, which may expose cells to a more chaotic environment, where critical proteins seep in and disrupt normal cellular functions. "The whole point of condensates is to separate biological reactions to help them work efficiently," Dr. Tyler explained, highlighting the perils of a leaky nucleolus that can ultimately lead to genomic instability, an early sign of aging.
Looking Ahead: Implications for Human Aging
The implications of this research extend far beyond yeast. The team plans to investigate the nucleolar influence on aging in human stem cells, which hold significant promise due to their unique ability to replace various types of cells as they age. By leveraging insights from this study, researchers hope to unlock new strategies for extending the lifespan and functionality of stem cells—potentially revolutionizing our understanding of aging.
As science continues to unveil the mysteries of cellular aging, could we be on the cusp of a breakthrough that will shift our approach to healthcare in old age? As we delve deeper into these cellular mechanisms, the answer may not be far off.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)