Transforming Metals into Marvels: The Breakthrough of Multimetallic Aerogels as Electrocatalysts
2024-12-09
Author: Amelia
Introduction
Imagine being able to transform high-density metals into an ultra-light aerogel! This seemingly bizarre concept was first introduced in 2009 by a research team led by Eychmüller, paving the way for the creation of all-metal-made aerogels, known as metal aerogels (MAs). These pioneering materials, forged by assembling metal nanoparticles in a meticulous manner, have captured the attention of scientists worldwide and established a new realm in materials science.
Multimetallic Aerogels: A Unique Approach
Of particular intrigue are multimetallic aerogels (MMAs), which combine more than one type of metal. What makes MMAs so special? They possess highly tunable properties that arise from the synergy of different metal elements, leading researchers to ponder whether these materials would inherit characteristics from each component, or if they would exhibit superior performance due to their interactions.
Enhanced Efficiency in Electrocatalysis
Recent studies show that MMAs often outperform single-metal aerogels in applications such as electrocatalysis. This enhanced efficiency stems from manipulating differences in electrical conductivity, lattice structures, and electronic configurations among various metals.
Research Advances in Synthesis
In our latest research published in Matter, titled "Manipulating Multimetallic Effects: Programming Size-Tailored Metal Aerogels as Self-Standing Electrocatalysts," we concentrated on the synthesis capabilities necessary for advancing these materials rather than solely their applications. Our investigation revealed the significant impact of multimetallic effects on the sol-gel process and resulting ligament sizes in MMAs.
Gravity-Driven Gelation Behavior
Five years ago, we discovered a remarkable gravity-driven gelation behavior in metallic systems, akin to precipitation processes. High-density metals, such as gold with a density of approximately 19.3 g/cm³, naturally settle over time to form a cohesive gel at the bottom of the container. Surprisingly, adding a lower-density metal, like silver (~10.5 g/cm³), to a gold-silver bimetallic system alters this behavior by slowing down sedimentation, thus prolonging gelation.
Controlling Ligament Sizes
Our findings, corroborated by numerous experiments with various metal combinations, not only provided a new method for controlling the sol-gel process of MMAs but also validated our proposed gravity-driven gelation mechanism.
The real game-changer, however, lies in our ability to control ligament sizes through multimetallic effects. Ligament size is critical because it influences numerous physicochemical properties of materials. Traditionally, this size was adjusted using initiators or ligands, which could contaminate the final product. Our research highlights that some metals, such as gold and silver, inherently tend to form larger ligaments, while alloy aerogels tend to possess smaller sizes.
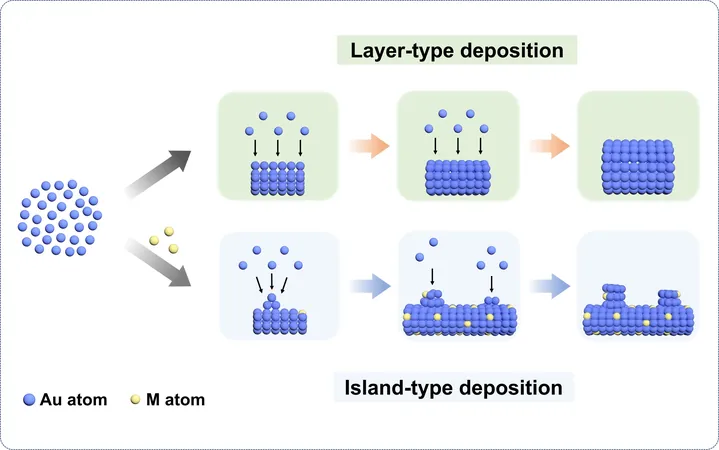
Innovative Research Findings
Through our rigorous studies, we introduced different types and quantities of auxiliary metals into primary metal systems, such as nickel into gold. Remarkably, even a 1% addition of auxiliary metals significantly reduced ligament sizes by 30% to 78% across gold, silver, and copper-based aerogels. This phenomenon can be explained through the atomic radius mismatch between the primary and auxiliary metals, which alters the deposition process during formation.
Non-Destructive Sedimentation Strategy
Given our discoveries and the gravitational behavior of gelation, we devised a pioneering, non-destructive sedimentation-based strategy to enhance the electrocatalytic performance of MMAs. This new technique avoids the structural damage that typically results from sonication methods used in previous studies on metal aerogels.
In our approach, carbon paper was strategically placed at the bottom of the reaction vessel to collect the settling metal aggregates. Over time, these aggregates accumulated on the carbon paper, forming an intact metal gel film, which we demonstrated with the Au-Pt system. This film was then tested as a working electrode for catalyzing alcohol oxidation reactions, achieving unprecedented efficacy for both methanol and ethanol oxidation.
Conclusion and Future Perspectives
In summary, our research offers a groundbreaking perspective on harnessing multimetallic effects to tailor the preparation and structure of MMAs. More importantly, it addresses the longstanding challenge of developing intact metal gel-based electrocatalysts capable of high-performance catalysis, potentially revolutionizing numerous applications in energy and environmental technologies.
Stay tuned as we continue to explore the forefront of materials science, with exciting developments that could redefine the future of energy solutions!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)