Unleashing the Power of Tobacco Plants: Revolutionary Strategies for Maximizing Recombinant Protein Production!
2024-09-25
Introduction to Plant Molecular Farming (PMF)
Modern biological science has reached an exciting pinnacle with the advent of plant molecular farming (PMF), a sophisticated technique harnessing plant biosynthetic machinery to produce a wide array of recombinant proteins, ranging from therapeutic enzymes to crucial industrial compounds. This innovative approach presents several compelling advantages over conventional methods such as microbial fermentation and mammalian cell culture. Notably, PMF boasts significantly lower production costs, high-yield outputs, and an absence of human pathogens and endotoxins that often plague traditional systems. The flexibility offered by plants also enables tailored protein production to meet specific needs.
Why Tobacco Plants?
Among various plant species, tobacco—specifically Nicotiana benthamiana and Nicotiana tabacum—stands out for its unique characteristics that make it particularly suitable for protein production. These plants have evolved to exhibit a compromised baseline immune response and a less robust RNA silencing pathway, which prevents the degradation of foreign RNA, thus facilitating quicker and more efficient recombinant protein synthesis. Furthermore, their relatively short life cycle and capacity for large biomass production significantly enhances their viability as bioreactors.
Challenges in Protein Localization
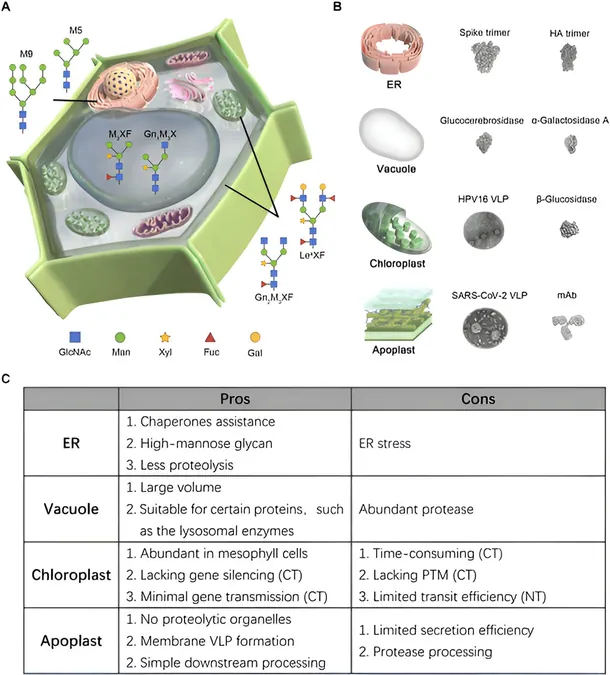
However, challenges remain in optimizing the subcellular localization of recombinant proteins within tobacco cells to ensure effective production. As a response to these complexities, a recent comprehensive review in BioDesign Research emphasized the need for targeted strategies to guide recombinant proteins to key subcellular compartments—including the endoplasmic reticulum (ER), vacuole, chloroplast, and apoplast.
Optimizing the Endoplasmic Reticulum (ER)
Dr. Shi-Jian Song, a leading researcher from the Chinese Academy of Agricultural Sciences, underscores the importance of this research, stating, 'Optimizing subcellular localization for individual target proteins is crucial for successful protein synthesis and its utilization in the pharmacological industry.' The ER is particularly notable for its role in protein processing, providing essential molecular chaperones that assist in protein folding while mitigating degradation risks. By incorporating specific signal peptides and retention sequences, researchers can enhance the accumulation of recombinant proteins in the plant's ER—a vital step for producing complex glycoproteins.
Managing Protein Load in the ER
However, as Dr. Inhwan Hwang notes, excessive protein loading in the ER could lead to stress responses and notably reduce yield. This highlights the necessity for careful management of expression levels during protein production.
Utilizing the Vacuole
On another front, the vacuole occupies a significant portion of the tobacco cell's volume and serves as an ideal compartment for storing recombinant proteins. Utilizing vacuolar sorting signals ensures specific targeting, although researchers must contend with the risk of proteolytic degradation within these compartments. Proteins engineered to withstand acidic environments and resembling human lysosomal proteins have shown greater success in vacuolar targeting.
Chloroplasts as a Production Platform
Moreover, chloroplasts represent an additional powerhouse for protein production, boasting the capability to store large quantities of proteins. While chloroplast transformation allows for stable gene expression with optimal folding conditions, the challenges in developing high-yield transgenic plants through this method remain formidable. Alternatively, nuclear transformation offers a more expedient approach, marrying increased protein production speed with effective control of biochemical modifications.
Exploring the Apoplast
Another promising avenue lies within the plant's apoplast—an area lying between the cell membrane and wall. This space is becoming increasingly recognized as a prime location for recombinant protein accumulation due to its ease of extraction. For larger protein complexes, conventional purification processes still apply, but innovative strategies, such as co-expressing protease inhibitors, are emerging to preserve protein integrity during extraction.
Future Prospects and Challenges
Though PMF has the potential to revolutionize the landscape of recombinant protein production, challenges such as adaptable production levels, maintenance of quality, and cost-effectiveness remain. Dr. Shi-Jian Song reinforces the need for strict adherence to established protocols, enhancing public engagement, and implementing robust safety measures to realize the full potential of transgenic plants in industrial applications.
Enhancing Tobacco Plant Chassis
Finally, improving the tobacco plant chassis to combat issues such as inefficient protease activity and organizational resource allocation while establishing a toxin-free environment will pave the way for significant advancements. The eventual commercialization of these biomanufacturing processes signals an exciting frontier for PMF, promising new horizons in pharmaceutical and industrial biotechnology.
Conclusion
Stay tuned as researchers continue to unlock the vast potential of tobacco plants in the world of recombinant protein production—could this be the future of sustainable biomanufacturing?









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)