Unveiling the Mysteries of Plant Cell Wall Construction: First-Ever Video Captures the Process!
2025-04-04
Author: Kai
Unveiling the Mysteries of Plant Cell Wall Construction
In a remarkable breakthrough for plant biology, researchers have captured the first-ever video showcasing the intricate process of plant cell wall construction. The cell wall, a protective layer surrounding plant cells, is made up of cellulose microfibrils intertwined with essential polysaccharides such as hemicellulose and pectin. While scientists have long understood the appearance of plant cells without their walls and in their fully formed state, this new research allows us to witness the complex wall-building process unfold in real-time.
Eric Lam, a plant biologist at Rutgers University and co-author of the groundbreaking study, noted, “We knew the starting point and the finishing point, but had no idea what happens in between.” What they discovered was far more intricate than the simplified representations found in traditional biology textbooks.
Challenges of Observation
Plant cells devoid of their walls, termed protoplasts, are exceedingly fragile and have historically been challenging to observe under a microscope for extended periods. They are also highly sensitive to light, making traditional microscopy techniques difficult to apply without damaging the samples.
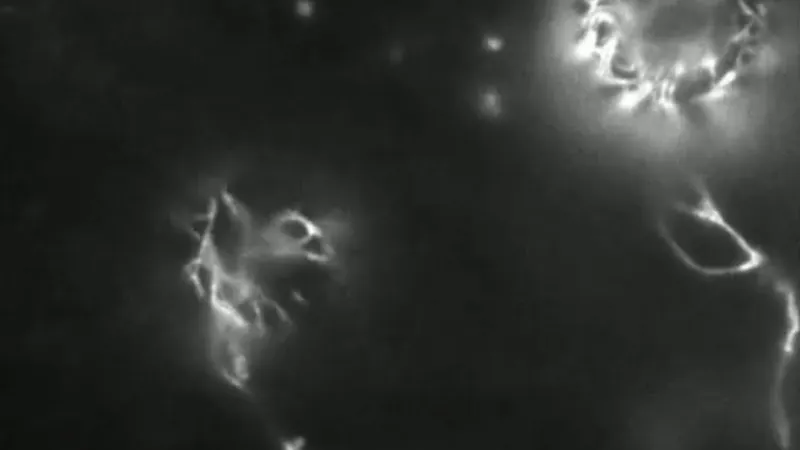
Moreover, tracking the process was hindered by the fact that cellulose fibers lack fluorescence, complicating visualizations. As Shishir Chundawat, another biologist involved in the research, explained, “You can’t see cellulose with traditional microscopy.” The common fluorescent markers used for labeling cellulose were either toxic to protoplasts or chemically bound to other compounds, rendering them unsuitable for use in live plant cells.
To overcome these obstacles, Lam and his team created a custom imaging platform. The protoplasts they focused on were sourced from Arabidopsis, a model plant closely related to cabbage and mustard. They utilized total internal reflection fluorescence microscopy (TIRFM), a technique that illuminates only a specific area of the sample, thereby minimizing phototoxicity. They also developed a non-toxic fluorescent marker, linking it to enzyme components capable of binding to cellulose.
The team designed a rigorous experimental environment, including a programmable LED light to control exposure and a temperature regulation system that maintained a steady 18°C (64°F). After a prolonged 24-hour observation period, the team was ready to record the dynamic wall-building activity of the protoplasts.
Unexpected Discoveries
What unfolded before their eyes diverged significantly from prior expectations. Chundawat described the initial findings: "We anticipated a straightforward stream of continuous polymer fibers, but the reality was much more complex." The researchers observed that very short cellulose fibers formed first and moved across the cell surface in a seemingly random manner, akin to a chaotic search for connections. It was only upon encounter and interaction with other short fibers that longer, intertwining strands emerged, ultimately assembling into the organized structure of the cell wall. “It was a two-step process,” acknowledges Chundawat, reflecting the depth of their unexpected discoveries.
These observations led to new questions about the mechanics of cellulose assembly. Is the process energy-driven, advancing in a specific direction, or is it influenced by random encounters? Eric Lam expressed interest in delving deeper into these mechanisms, stating, “We don’t know, but we will find out.”
The initial time-lapse only captured cellulose fibers post-assembly, hinting at the involvement of various enzymes crucial for the biosynthesis and transport of cellulose that remain unelucidated in this footage. Future investigations will focus on three-dimensional recordings using fluorescent tags attached to these enzymes, allowing researchers to pinpoint which proteins oversee cellulose biosynthesis.
The Road Ahead: Enhancing Crop Yields
With a powerful imaging platform that can document the entire process of cell wall construction from beginning to end, the team plans to explore how manipulating different genes and biochemical pathways might affect wall assembly. “It’s like in a car—by removing one piece of the system at a time, you can see how the performance is altered," Lam likened. The ultimate goal is to identify key genes that can be fine-tuned to amplify biomass yield significantly.
By enhancing the output from agricultural land, this research could pave the way for more resilient crops, ultimately lowering the costs associated with the production of biochemicals and biofuels. Chundawat concluded with an optimistic vision, "We could potentially revolutionize agriculture with this knowledge."
This monumental study not only sheds light on the mechanisms of cellular construction in plants but also holds promising implications for future agricultural innovations and sustainability.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)