Ancient Protein Structure Unlocks Secrets of Early Life Evolution
2024-10-03
Author: Daniel
Introduction
In a groundbreaking study that redefines our understanding of life's origins, two researchers from RIKEN have uncovered an ancient protein fold that may have played a pivotal role in the early molecular evolution and diversification of life on Earth. This remarkable discovery, absent in contemporary proteins, shines a new light on how complex biological systems emerged from simpler precursors.
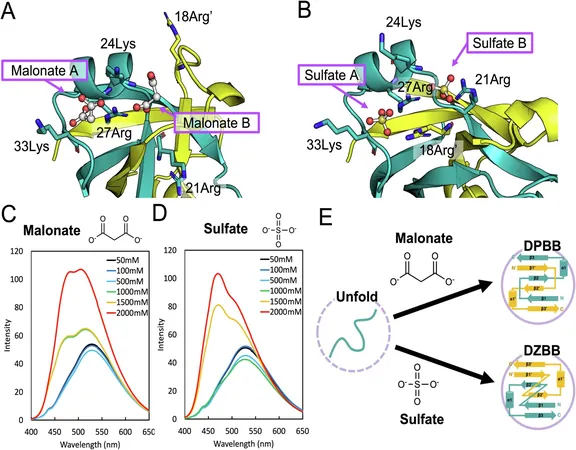
The Discovery of the Double-Zeta β-Barrel
The proteins responsible for critical biological functions, including gene expression and protein synthesis, are characterized by distinct structural formations, notably the β-barrel folds. Despite the acknowledgment of these folds, the evolutionary linkages between them remained elusive—until now.
Research Insights by RIKEN Biologists
RIKEN biologists Shunsuke Tagami and Sota Yagi have simulated what they term the double-zeta β-barrel (DZBB), an ancient folding topology that may serve as an evolutionary missing link. According to Tagami, "This revelation simplifies our understanding of the evolutionary relationships among diverse proteins in unexpected ways."
Structural Characteristics of DZBB
The DZBB structure resembles a compact cylinder composed of interwoven protein strands, exhibiting a unique origami-like ability to transform into various essential protein configurations with mere modifications. This flexibility positions the DZBB assemblies as a crucial blueprint for molecular evolution.
Experimental Journey Through Evolution
By employing synthetic biology techniques in their laboratory experiments, Tagami and Yagi traced the evolutionary journey from a primitive fold found in DNA and RNA polymerases—crucial enzymes for genome replication and gene transcription—to the more complex folds seen in modern ribosomal proteins, which are vital for cellular protein synthesis.
AI Limitations in Protein Structure Prediction
Their research indicates that this evolutionary transition necessitated the intermediary DZBB structure, which could not be predicted through computational approaches, including advanced machine-learning algorithms. This limitation highlights a significant challenge within current AI models that struggle to identify intricate protein architectures without experimental verification. Tagami noted, "While AI can provide insights, experimental validation remains essential for uncovering the unexpected."
Implications for Understanding Primordial Proteins
The implications of this discovery extend to the understanding of primordial proteins and their role in governing genetic processes. The metamorphic characteristics of DZBB, allowing it to stabilize in multiple forms under varying conditions, suggest that early life molecular machinery may have rapidly diversified, akin to the sudden explosion of animal species during the Cambrian period.
A Thought-Provoking Question
However, this breakthrough also poses a thought-provoking question: If the DZBB fold was instrumental in the evolution of molecular machines that regulate genetic information, why has this topology vanished from modern proteins? Tagami speculates, "DZBB may represent a transient protein form, emerging solely during a critical evolutionary phase between ancient simple forms and more complex modern proteins."
Conclusion
As researchers delve deeper into the mysteries of molecular evolution, findings like these could reshape our understanding of life's evolutionary narrative, providing foundational knowledge about the building blocks that shaped the early biosphere. This study, recently published in *Nature Communications*, paves the way for further exploration into the fascinating world of ancient proteins and their profound impact on the lineage of life.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)