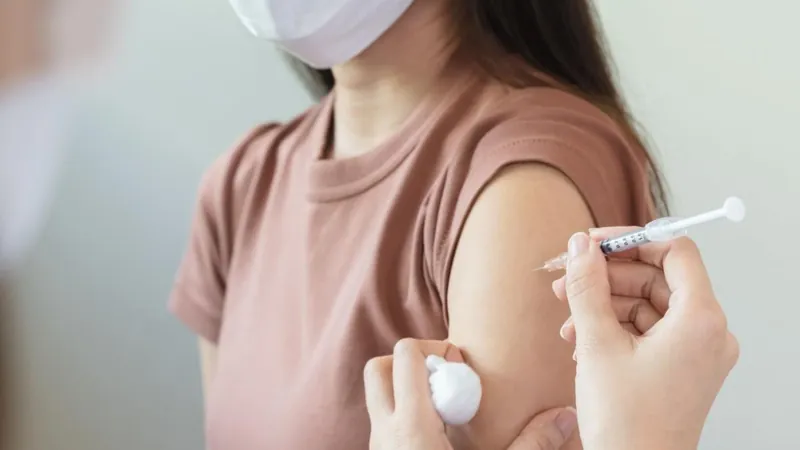
Bavarian Nordic Teams Up With Serum Institute of India to Boost Mpox Vaccine Production
2024-12-17
Author: Li
Bavarian Nordic and Serum Institute of India Agreement
Bavarian Nordic has entered into a pivotal licensing and manufacturing agreement with the Serum Institute of India (SII) for its mpox vaccine, known as MVA-BN. This partnership is set to revolutionize vaccine accessibility in India by facilitating the local manufacturing of the vaccine through a comprehensive technology transfer.
Manufacturing and Distribution Rights
Under this agreement, SII will not only be authorized to sell and distribute MVA-BN across India but will also handle contract manufacturing for Bavarian Nordic once necessary regulatory approvals are secured. This collaboration is strategically designed to enhance global production capacity during mpox outbreaks, ensuring the availability of vaccines where they are most needed.
Innovative Financial Model
Notably, the financial framework of this partnership operates on a innovative profit-sharing model, eliminating the need for upfront payments or milestone costs from either party. SII is responsible for acquiring and maintaining regulatory approvals for the vaccine in India, while both companies will share the costs associated with the technology transfer.
Global Aspirations
Bavarian Nordic's initiative doesn’t stop in India; the company is actively seeking additional partnerships to ensure equitable access to the MVA-BN vaccine worldwide. One of their key focuses is establishing collaborations with local manufacturers in Africa, aiming to reach a broader demographic and address the pressing health needs in the region.
Leadership Thoughts
Paul Chaplin, the president and CEO of Bavarian Nordic, expressed enthusiasm for the new agreement, stating, “We are excited to kick off this licensing and manufacturing agreement for MVA-BN as an mpox vaccine, which marks a significant advancement in our goal to increase vaccine access for all communities.”
Regulatory Developments
In a significant development, the European Medicines Agency (EMA) approved the usage of Bavarian Nordic's mpox vaccine for adolescents in September 2024, potentially broadening its reach to younger populations, particularly in Africa.
CEO Statements
Adar Poonawalla, CEO of the Serum Institute of India, emphasized the importance of this collaboration, stating, “Our mission has always been to deliver high-quality, affordable vaccines worldwide. The recent mpox outbreak highlights the urgent need for a prompt and organized response. By leveraging our production capabilities, we aim to improve epidemic preparedness and ensure that vulnerable populations receive life-saving vaccines, ultimately reducing the global burden of mpox.”
Public Health Implications
This alliance not only promises to enhance local manufacturing but also indicates a significant step towards a more responsive and robust public health infrastructure capable of mitigating future health crises.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)