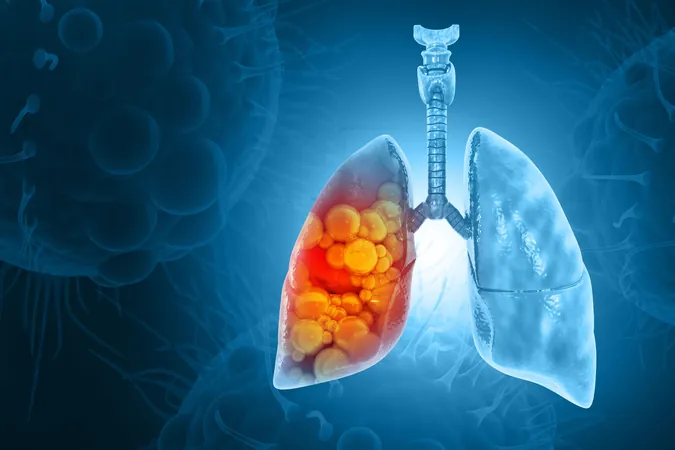
Bizengri Emerges as a Game-Changer for NRG1 Fusion-Positive Cancers, Including Aggressive Lung Cancer
2025-04-03
Author: Siti
Introduction
Groundbreaking research published in the prestigious New England Journal of Medicine in February 2025 highlights the promise of Bizengri (zenocutuzumab), a cutting-edge bispecific antibody treatment for patients battling advanced NRG1 fusion-positive cancers.
FDA Approval and Treatment Landscape
Following accelerated FDA approval in December 2024, Merus’s Bizengri is now a potential lifeline for adults facing advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC) or pancreatic adenocarcinoma with NRG1 gene fusions. This approval comes as a beacon of hope for patients whose conditions have worsened despite prior treatments.
Clinical Trial Results
The pivotal eNRGy study—a comprehensive trial involving 94 adults with either NRG1 fusion-positive NSCLC or pancreatic cancer—yielded promising results. Among the participants, a 33% overall response rate (ORR) was noted for NSCLC and a remarkable 40% ORR for pancreatic cancer, demonstrating a response duration spanning from 3.7 to 16.6 months.
Understanding NRG1 Fusion-Positive Cancers
The NRG1 protein plays a vital role in normal tissue development; however, when mutations lead to gene fusions, it can trigger aggressive cancer growth. Although such fusions occur in fewer than 1% of solid tumors, they are increasingly prevalent in certain forms of lung and pancreatic cancer.
Mechanism of Action of Bizengri
Bizengri is designed to tackle this challenge head-on. By targeting the interaction between the HER2 and HER3 proteins found on cancer cells, it inhibits the overactive signaling that contributes to unchecked cell proliferation. Specifically, Bizengri prevents the NRG1 fusion proteins from binding to HER3, effectively disrupting the signaling pathways that fuel tumor growth.
Expanded Clinical Trial Findings
A recently expanded phase 2 clinical trial, led by Dr. Alison M. Schram, an oncologist and early drug development expert from Memorial Sloan Kettering Cancer Center in New York, supports earlier findings with further validation. This study, which included 204 patients with diverse NRG1 fusion-positive solid tumors, demonstrated a 30% therapeutic response rate among those with measurable disease. The median treatment response lasted an impressive 11.1 months.
Variability in Treatment Response
Significantly, the treatment responses varied across various cancer types, with 29% of NSCLC patients and an encouraging 42% of pancreatic cancer patients showing positive outcomes. Overall, the trial noted a median progression-free survival of 6.8 months, raising hopes within the oncological community.
Experts Weigh In
Dr. Schram shared her enthusiasm about these findings, stating, “This study is a milestone in recognizing NRG1 fusions as targetable oncogenic drivers in cancer, and it paves the way for potential targeted therapies specifically designed for this patient demographic.”
Safety of Bizengri
As for safety, the drug's adverse effects were generally mild, with diarrhea, fatigue, and nausea being the most reported issues. Infusion-related reactions occurred in 14% of patients, but encouragingly, only a single case required interruption of therapy due to treatment-related complications.
Conclusion
With the devastation caused by NSCLC and pancreatic cancer and the limited options available for NRG1 fusion-positive patients, Bizengri offers an innovative treatment avenue. “Given the notable efficacy of zenocutuzumab in treating these challenging cancers, its approval marks a significant advancement for patients who desperately need new treatment options,” Dr. Schram concluded.
Future Outlook
As researchers continue to explore the full potential of Bizengri, the future looks brighter for patients grappling with these formidable cancers.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)