Breaking Ground in Water Oxidation Technology: A Leap Toward Sustainable Energy Solutions!
2024-12-18
Author: Siti
Introduction
As the world increasingly seeks sustainable and renewable energy options, the development of efficient methods for clean energy production has become an urgent priority. Picture a future where the energy cascading through our homes and cities stems from one of the Earth's most plentiful resources—water.
The Breakthrough in Photochemical Water Oxidation
A breakthrough is underway in photochemical water oxidation, a revolutionary process that harnesses light to split water molecules, yielding oxygen and facilitating the generation of clean energy. While water oxidation presents a wealth of possibilities, the intricacies surrounding the catalytic activity and the variances among different catalysts still remain a mystery.
Research at the Institute of Science Tokyo
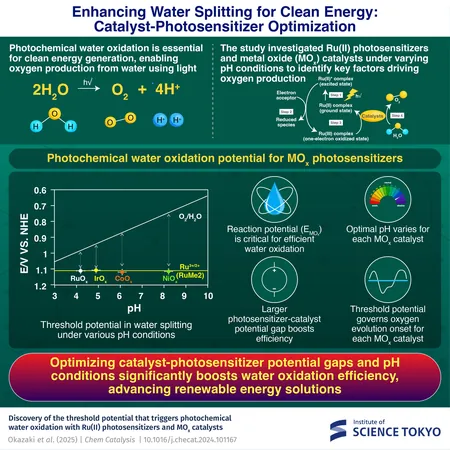
Researchers from the Institute of Science Tokyo, spearheaded by the innovative Assistant Professor Megumi Okazaki, are diving deep into the characteristics that fuel this crucial process. Their recent study, published in the esteemed journal Chem Catalysis, sheds light on the influential factors of water splitting, especially focusing on Ru(II) photosensitizers, metal oxide (MOx) catalysts, and the impact of pH levels.
Methodology and Discoveries
In this groundbreaking research, the team meticulously examined the performance of Ru(II) photosensitizers when combined with various MOx catalysts under differing pH environments. They introduced a pioneering approach allowing them to estimate the reaction potential (EMOx) of catalysts without requiring complex electrochemical equipment, streamlining the efficiency assessment.
Significant Findings
The gathered data led to groundbreaking discoveries, mapping out the thresholds for when oxygen evolution begins and assessing how the potential differences between the photosensitizer and the catalyst affect overall efficiency. According to Okazaki, "Reaction potential (EMOx) is pivotal in the water oxidation process, visually illustrating a driving force for reactions that has never been measured under operational conditions before."
Tailoring Reaction Environments
The study further illuminated how the onset conditions of pH vary among different MOx catalysts, underscoring the necessity for tailored reaction environments for each individual catalyst. The researchers put significant emphasis on the ‘threshold potential’—the exact point at which oxygen production initiates, marking the commencement of the reaction.
Improving Water Oxidation Efficiency
The researchers confirmed that by fine-tuning both reaction potential and pH conditions, they could drastically improve water oxidation efficiency. Identifying optimal conditions for each catalyst lays down a strategic pathway for designing more effective and practical energy systems.
Accessibility and Future Impact
By simplifying the method to estimate reaction potentials, Okazaki notes, "We're making this research more accessible and cost-effective. This breakthrough could revolutionize how we develop and select catalysts, accelerating advancements toward more efficient, sustainable energy solutions."
Conclusion
These findings mark vital progress in our journey toward a sustainable future. By refining reaction conditions, scientists have the potential to craft more efficient systems for generating clean energy. This pivotal shift not only diminishes our dependency on fossil fuels but also broadens access to renewable energy technologies on a global scale. Furthermore, the innovative potential estimation method stands to reshape catalyst design practices, fast-tracking progress in this essential field of research.
Don't miss out on how this cutting-edge research could change the landscape of energy production forever!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)