Breakthrough Discovery: Mini-Hairpin Peptide Disrupts Protein Synthesis in E. coli!
2025-04-18
Author: Daniel
The Crucial Role of Proteins in Cellular Function
Proteins are the very backbone of cellular life, driving everything from structural integrity to essential metabolic processes. Disruptions in protein synthesis can cascade into significant cellular dysfunction, making understanding this process paramount.
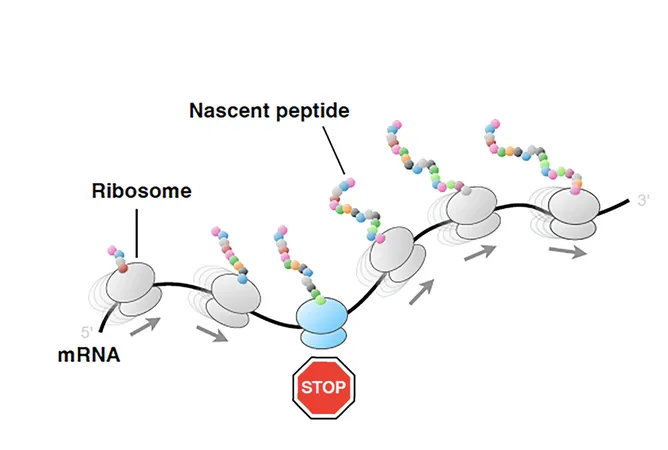
Ribosomes: The Unsung Heroes of Protein Creation
At the heart of protein synthesis lies the ribosome, a complex molecular machine that decodes messenger RNA (mRNA) and assembles amino acids to form proteins. Recent studies have unveiled that ribosomes do more than just stitch together polypeptide chains; they also play a crucial role in regulating this intricate process through interactions with both the nascent peptide and various regulatory factors.
Translation Arrest: A Bacterial Survival Mechanism
But what happens when protein synthesis goes awry? Translation arrest—a phenomenon where the ribosome halts elongation—is often triggered by environmental factors such as nutrient availability and the presence of inhibitors like antibiotics. Understanding this mechanism is critical, especially as ribosome arrest peptides (RAPs), which can cause translation shutdown, have remained largely enigmatic.
Unraveling the Mystery of Ribosome Arrest Peptides
In a groundbreaking study, Dr. Yuhei Chadani and a dedicated team from Okayama University and Tokyo University embarked on a mission to identify and explore RAPs in *Escherichia coli* (E. coli). Their aim? To dive into the structural diversity of nascent peptides formed in the ribosomal tunnel and understand their roles in translation regulation.
The Impact of TnaC: A Closer Look at RAP Activity
The research team focused on TnaC, a tryptophan-dependent RAP known for suppressing cell growth and inducing toxicity. Analyzing 38 small open reading frames (sORFs), they discovered that 18 of these sequences significantly hindered bacterial growth. Intriguingly, these cytotoxic effects appeared unrelated to the regulation of downstream genes.
Cold Shock Proteins: The Body’s Response to Stress
Bacteria express cold shock proteins (CSPs) when translation elongation is inhibited by stressors. The researchers performed a comparative proteomic analysis, uncovering that TnaC and antibiotic-induced translation arrest are linked to CSP expression. Remarkably, overexpression of 12 sORFs also resulted in heightened CSP levels.
Stalling the Ribosome: The Role of PepNL and NanCL
In a significant discovery, ribosome profiling revealed that the peptides "PepNL" and "NanCL" triggered translation arrest in *E. coli*. Further structural analysis showcasing how PepNL adopts a mini-hairpin conformation in the ribosomal exit tunnel brought new insights into the mechanisms behind this process.
Dramatic Structural Changes Ahead!
Typically, when a ribosome encounters a stop codon, peptide release factors (RF) release the peptide chain from tRNA. However, Dr. Chadani’s team found that the presence of the PepNL nascent peptide led to structural changes in RF2, rendering it inactive. Uniquely, the folding of PepNL does not necessitate an arrest inducer, unlike many other RAPs, allowing it to adaptively recognize stop codon read-throughs.
Implications for Future Research
This study not only identified two novel RAPs in *E. coli* but also shed light on the unique structural mechanisms at play in gene regulation and environmental adaptation. With these revelations, the pathway to innovating biosensors and enhancing protein synthesis could be on the horizon.
Published Insights in Nature Communications!
These remarkable findings have been detailed in the prestigious journal Nature Communications, illuminating a new frontier in understanding how peptide structures can impact bacterial life. Stay tuned as researchers continue to unravel the complexities of protein synthesis!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)