Breakthrough Discovery: New Genetic Mutation Could Revolutionize Cancer Treatments
2024-12-27
Author: Daniel
Breakthrough Discovery: New Genetic Mutation Could Revolutionize Cancer Treatments
Researchers at UT Southwestern Medical Center have made a groundbreaking discovery of a genetic mutation that significantly slows the growth of melanoma and potentially other types of cancer. This remarkable finding could pave the way for new and improved treatments aimed at enhancing the effectiveness of existing cancer immunotherapies.
Dr. Hexin Shi, Assistant Professor in the Center for the Genetics of Host Defense and Immunology at UT Southwestern, emphasizes that this discovery introduces an entirely new therapeutic target. “Our findings suggest a completely new type of therapeutic target that could someday be used to suppress a wide range of cancers,” he stated, highlighting the potential this research has for the future of cancer treatment.
Co-leading the study, Dr. Bruce Beutler, a Nobel Prize laureate in Physiology or Medicine, alongside Dr. Shi, has made seminal contributions to our understanding of how the immune system can react to infections, which could now be leveraged against cancer. He is part of the Cellular Networks in Cancer Research Program at the Harold C. Simmons Comprehensive Cancer Center at UTSW, where this innovative research is taking place.
Historically, while many oncogenes have been linked to cancer progression when mutated, scientists have surmised that certain mutations confer resistance to cancer. However, identifying these protective mutations in humans has been challenging due to the lack of obvious distinctions in those who carry them.
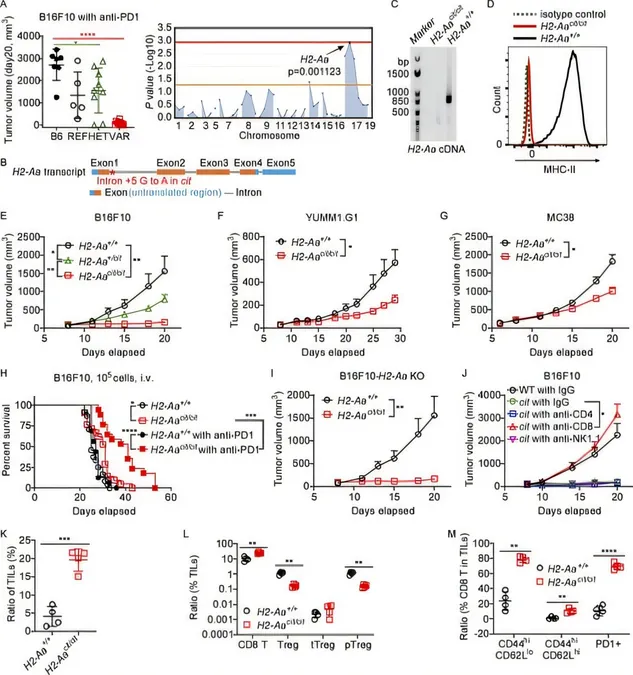
In an effort to uncover these elusive tumor-resistant genes, the research team created a series of genetically modified mouse models. Utilizing a technique called automated meiotic mapping (AMM), they meticulously traced unusual characteristics in these mutant mice back to their causative mutations. Their search culminated in the identification of a gene known as H2-Aa. Intriguingly, mice missing this gene showed remarkable resistance to melanoma, indicating that the absence of the H2-Aa protein dampens tumor growth.
But what makes this discovery particularly thrilling is how the mutation alters the immune response. H2-Aa plays a vital role in producing MHC class II proteins, essential for the immune system in differentiating between self and non-self proteins. By genetically engineering the mice to eliminate H2-Aa specifically from the immune cells known as dendritic cells, the researchers found that this targeted removal was sufficient to replicate the protective effects observed with completely H2-Aa-deficient mice.
Remarkably, tumors in these mutant mice showed a significantly greater presence of immune cells that attack cancer, specifically CD8 T cells, and a marked reduction in regulatory T cells that typically suppress anti-cancer activity.
In seeking a potential pharmaceutical application, the researchers engineered a monoclonal antibody against H2-Aa. When tested on mice with melanoma tumors, the antibody exhibited a notable anti-cancer effect, especially when used in conjunction with checkpoint inhibitors—offerings of the latest immunotherapy approaches. Dr. Beutler noted the possibility that human versions of these monoclonal antibodies might yield similar therapeutic benefits, suggesting a promising pathway for future clinical trials.
With statistics revealing that between 50% to 66% of melanoma patients do not respond to existing checkpoint inhibitors, this novel research could be a game-changer, potentially enhancing treatment efficacy and offering hope to a vast number of patients facing aggressive cancers.
As researchers continue to explore this promising avenue, the implications for transforming cancer treatment landscape could be profound, potentially making previously ineffective therapies accessible to millions of patients worldwide.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)