Breakthrough Discovery Uncovers Fibrosis Driver in Crohn’s Disease!
2024-12-18
Author: Wei Ling
Breakthrough Discovery Uncovers Fibrosis Driver in Crohn’s Disease!
In a significant advancement for understanding Crohn's disease, researchers have pinpointed a critical factor behind the alarming development of fibrosis, a condition that leads to scar-like tissue build-up in the intestines. This chronic inflammatory bowel disease (IBD) often results in life-altering complications, including the narrowing of the intestines and the possibility of devastating bowel obstructions and infections, necessitating surgical interventions for most patients.
Historically, the lack of effective treatments for intestinal fibrosis has been attributed to a limited understanding of its cellular mechanisms. However, a groundbreaking study led by post-doctoral researcher Ju-Hyun Ahn, PhD, and Janelle Arthur, PhD, an associate professor at the UNC School of Medicine, has shed light on the interactions between gut bacteria and host cells that drive this process. Their findings, published in the esteemed journal *Cell Host & Microbe*, present a promising avenue for targeted therapies for Crohn's disease management.
"Our research reveals that a small molecule called yersiniabactin can alter host intracellular zinc levels, leading to the activation of macrophages that contributes to fibrosis," explained Ahn. This discovery highlights that monitoring macrophage activation and zinc imbalances could open doors to identifying high-risk patients and tailoring new treatments for this debilitating condition.
Macrophages, a type of white blood cell, play a crucial role in immune response by engulfing pathogens and cellular debris. They rely heavily on zinc to carry out their functions effectively. In the context of IBD, when these immune defenders engage in fighting infections, they can become dysregulated. This dysfunction is often linked to the activation of a protein called hypoxia-inducible factor 1-alpha (HIF-1α), which, when overly activated, can trigger excessive fibroblast activity, leading to unwanted tissue growth and, ultimately, fibrosis.
The researchers focused on adherent-invasive E. coli, a microbe that can exacerbate Crohn’s disease under certain conditions. Previous work established that yersiniabactin produced by these bacteria induces fibrosis; however, the team sought to clarify the connection between yersiniabactin-bound metals and macrophage disruption. Using advanced gene editing techniques like CRISPR-Cas9, they confirmed that yersiniabactin indeed siphons zinc from macrophages, impairing their function and activating the HIF-1α pathway, which fuels the expression of pro-fibrotic genes.
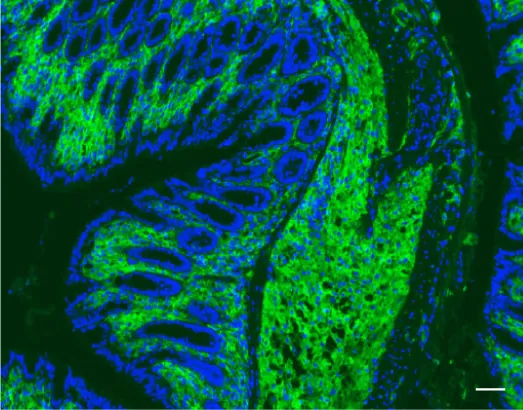
In a striking observation, the researchers found an abundance of HIF-1α+ macrophages in fibrotic tissues from patients with IBD undergoing surgery. Utilizing a fluorescent dye for tracking these macrophages, they established that this activation occurs specifically at sites of inflammation, reinforcing the notion that gut bacteria play a pivotal role in managing nutrient metals that influence fibrosis through interactions between macrophages and fibroblasts.
With these findings in tow, the Arthur lab plans to further investigate whether the presence of HIF-1α+ macrophages can be used as a biomarker for fibrosis in biopsies from future Crohn’s patients. They will also explore how different aspects of the microbiome, specifically those that produce yersiniabactin or compete for zinc, might be leveraged to predict which patients are at a higher risk for developing fibrosis.
Although these groundbreaking results pave the way for future efforts to combat fibrosis in Crohn's disease, more research is required to fully unravel the intricate role of HIF-1α+ macrophages and their implications. As Ahn embarks on continued molecular studies and additional mouse model research, backed by a fellowship from the Crohn's and Colitis Foundation, the hope is to develop innovative strategies that can transform patient care for those living with this challenging disease.
Stay tuned as we follow this compelling narrative and its potential impact on millions suffering from Crohn's disease!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)