Breakthrough in Battery Technology: New Technique Unveils Secrets of Electrolyte Interfaces!
2024-10-07
Author: Siti
Introduction
Batteries are the backbone of modern technology, powering everything from smartphones to electric vehicles. A recent breakthrough by researchers at Penn State and industry partners is set to transform our understanding of battery performance by focusing on the pivotal interface between electrodes and electrolytes. Their groundbreaking work, published in the Journal of the American Chemical Society, unveils a new high-resolution method to analyze this crucial boundary, potentially unlocking new pathways to enhance battery efficiency and longevity.
The Role of Electrodes and Electrolytes
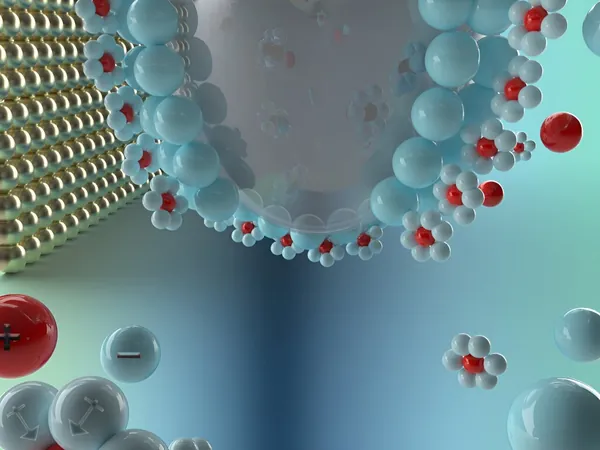
At the heart of every battery lies its electrodes: the anode (the negative electrode) and cathode (the positive electrode). These components are bathed in an electrolyte, which serves as the medium that facilitates ion movement, thereby allowing electrical current to flow. The performance of batteries is significantly influenced by the electrode-electrolyte interface—the area where solid meets liquid. Understanding how ions and solvent molecules interact at this interface is essential for improving battery design and performance.
Understanding the Electric Double Layer
The electric double layer (EDL) is particularly critical in this context. This nanometer-scale structure dictates ion migration and electron transfer, which are fundamental to electrochemical reactions within the battery. Graduate research assistant Jianwei Lai emphasizes that "the EDL governs the essential processes that enable battery functionality. Studying this double layer can directly impact battery performance."
Challenges in Observing the EDL
However, observing the EDL has always posed challenges due to its dynamic nature; it alters in response to varying voltage levels, akin to how traffic patterns shift during peak hours. These changes can lead to inefficiencies, such as ions becoming trapped and hindering smooth electrical flow, effectively resulting in 'traffic jams' within the battery.
Advancements in Measurement Techniques
Previously, researchers relied on theoretical models and traditional measurement techniques like voltammetry and electrochemical impedance spectroscopy, which provided indirect insights but lacked precision. To tackle these limitations, Lai and the research team have introduced an enhanced version of electrocapillarity, a technique that measures changes in surface tension at the electrode-electrolyte interface when voltage is applied.
Innovative Methodology and Findings
Utilizing advanced sensors and analytics, their innovative approach can discern not only overall interfacial tension but also the specific distribution of ions and the potential variations present at the interface. This high-resolution methodology has astonishingly improved data resolution by 50 to 100 times compared to conventional techniques, allowing researchers to visualize the double layer structure at individual voltage levels.
Insights from Zinc Battery Electrolytes
In their exploration of zinc battery electrolytes—an eco-friendly and cost-effective option gaining traction—the researchers made intriguing discoveries. They observed that chloride ions play a pivotal role in guiding zinc ions to the optimal locations at the electrode's surface, thus enhancing charging efficiency. "Our findings indicate a unique interaction that enables faster movement of zinc ions, thus improving battery efficiency during charging and discharging cycles," said Lai.
Future Implications and Conclusion
This newfound insight into the intricate dance of ions at the electrode-electrolyte interface means that scientists can now comprehend how various components impact battery performance. This technique can serve as a vital tool for designing superior electrolytes that pave the way for advanced energy storage solutions.
As Lai notes, understanding this interface is crucial for engineering better, more reliable electrolytes that can meet the demands of future clean energy technologies. With the modernization of electrocapillarity, researchers now possess an unprecedented ability to study and optimize the critical processes within batteries.
In an era where energy storage is more crucial than ever, this discovery could lead to revolutionary advancements, enhancing the efficiency and lifespan of batteries that power our everyday lives. The future of battery technology is bright, and with continued innovation, we may soon see significant leaps forward in how we store and use energy.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)