Breakthrough in CO₂ Reduction: Scientists Enhance Ethylene Production Using Innovative Boron Frameworks
2024-10-10
Author: Nur
Breakthrough in CO₂ Reduction: Scientists Enhance Ethylene Production Using Innovative Boron Frameworks
In an exciting advancement for sustainable chemistry, researchers have developed a new class of lightweight metal-organic frameworks known as boron imidazolate frameworks (BIFs), which have shown great promise in the electrocatalytic reduction of carbon dioxide (CO₂) to ethylene (C₂H₄). These crystalline BIFs mimic the structure of zeolite molecular sieves and feature a unique combination of covalent (B–N) and metal coordination bonds (M–N), positioning them as an exceptional crossover between traditional metal-organic frameworks (MOFs) and covalent organic frameworks (COFs).
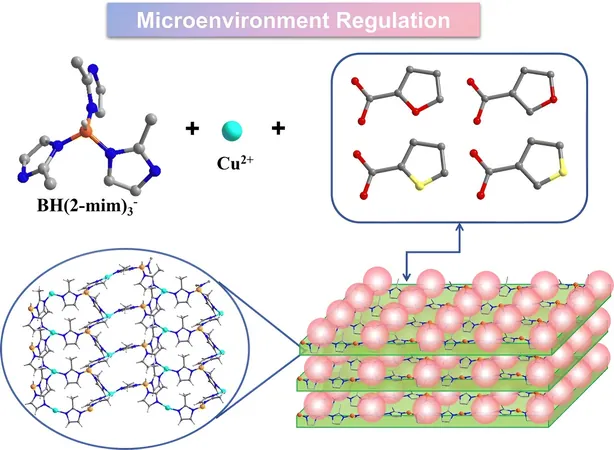
In a pioneering study published in the esteemed journal Chemical Communications, Professors Zhang Jian and Zhang Haixia from the Fujian Institute of Research on the Structure of Matter at the Chinese Academy of Sciences unveiled a series of isostructural two-dimensional (2D) BIFs. By introducing second carboxylic ligands with similar structures, they successfully altered the coordination microenvironment, leading to enhanced efficiency in converting CO₂ into ethylene through electrocatalytic processes.
The researchers created four distinct crystalline structures, labeled BIF-151 to BIF-154, each possessing the same fundamental framework and metal coordination environment, enabled by the unique KBH(mim)₃ ligand. They further discovered the effectiveness of four types of monocarboxylate ligands with varied substituent elements and positions, acting as flexible attachments to these layered frameworks.
Remarkably, these four BIFs exhibited exceptional stability across varying pH levels and solvent conditions, underscoring their potential for practical applications. Moreover, the electrocatalytic tests revealed that each crystal demonstrated notable catalytic activity towards CO₂ reduction, with specific selectivity for C₂H₄ production. Interestingly, while all four configurations facilitated CO₂ reduction, their respective activity and selectivity varied significantly.
These groundbreaking findings emphasize that the catalytic performance is intricately linked not just to the active metal sites but also heavily influenced by the microenvironment created by the surrounding ligands. Factors such as the composition and the spatial arrangement of substituent elements play a crucial role in determining the efficiency of CO₂ conversion.
In conclusion, this research not only propels our understanding of CO₂ electroreduction but also paves the way for the development of more effective catalysts that can help combat climate change by transforming greenhouse gases into valuable hydrocarbons. With the world urgently seeking sustainable solutions, innovations like these strike at the heart of the transition to a greener and more environmentally friendly future. Stay tuned for more updates on this promising field of electrochemistry!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)