Breakthrough in COVID-19 Treatment: New Oral Drug Outshines Paxlovid Against Resistant Strains!
2025-04-02
Author: Li
Introduction
Researchers at Rutgers Health have unveiled an exciting new oral antiviral drug candidate for COVID-19, dubbed Jun13296, that shows the potential to address significant shortcomings of Paxlovid, the currently most prescribed oral treatment.
Mechanism of Action
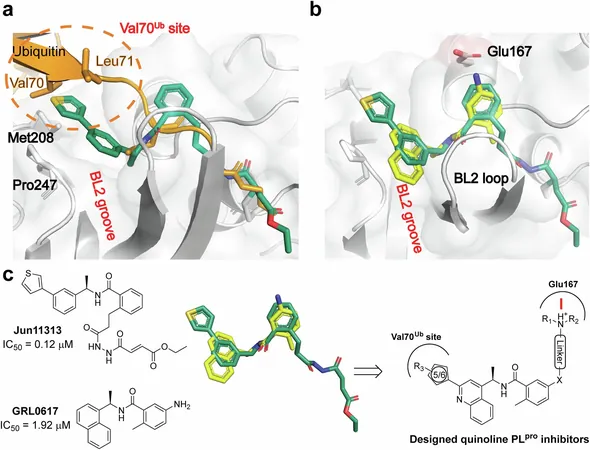
Unlike Paxlovid, which combines two compounds for its effect, Jun13296 operates independently by targeting a distinct viral protein—specifically, the papain-like protease (PLpro). This revolutionary approach not only enhances its effectiveness but also addresses the critical issue of drug interaction side effects that plague so many patients.
Efficacy and Testing
“Jun13296 is a significant advancement; it's more potent than our initial compound,” said Jun Wang, the study's senior author and a professor at Rutgers University’s Ernest Mario School of Pharmacy. In laboratory tests, Jun13296 has shown remarkable efficacy, providing a staggering 90% protection in animal models, and it does so at just one-third the dose of the initial drug.
Adverse Drug Interactions
One of the main hurdles for patients at high risk for severe COVID-19—many of whom are already on medications for conditions such as high blood pressure or diabetes—is the risk of adverse drug interactions when taking Paxlovid. Wang highlighted that this new drug has been crafted to mitigate those risks.
Resistance and Survival Rates
Laboratory analyses of Jun13296 confirm its effectiveness against Paxlovid-resistant variants, sparking hope for a wider range of treatment options. “Our studies show that it retains powerful inhibition against all tested virus variants,” Wang noted with optimism. Mice treated with Jun13296 exhibited a five-day survival rate of 90%, compared to only 40% for the previous iteration of the drug and a shocking 0% for untreated subjects.
Inflammation and Side Effects
The reduction of inflammation and viral load in the lungs was particularly notable. Jun13296 proved effective at 75 milligrams per kilogram, showcasing significantly better performance compared to the first-generation candidate, which only had moderate efficacy at the same dosage. This could mean fewer side effects and a safer course of treatment for patients.
Compatibility with Other Medications
What sets Jun13296 apart even further is its compatibility with other medications—initial tests reveal no inhibition of key drug-metabolizing CYP450 enzymes, allowing for safer co-administration with existing treatments.
Future Steps and Funding Needs
The road to human clinical trials is not without its challenges, however. Wang emphasized the need for significant funding, estimating costs in the tens of millions of dollars. The team aims to collaborate with pharmaceutical companies or nonprofit organizations to secure the necessary resources to push this promising drug through preclinical studies and onto FDA applications.
Pandemic Preparedness and Broader Applications
As COVID-19 continues mutating and presenting new variants that challenge existing treatments, Wang asserts the necessity for a diverse arsenal of therapies for pandemic readiness. Developing multiple treatment options is critical not just for immediate needs, but also for future contingencies if similar threats emerge.
Conclusion
Moreover, the methodologies refined by this research team may well extend beyond COVID-19, with applications for tackling other infectious diseases such as influenza and enteroviruses. Stay tuned: This revolutionary development could change the landscape of COVID-19 treatment and mark a significant leap forward in our ongoing battle against viral infections!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)