Breakthrough in Electrochemistry: Revolutionary Palladium Hydride Nanoparticles Discovered!
2024-11-19
Author: Yu
Introduction
In an exciting advancement for the energy sector, researchers at the Beckman Institute for Advanced Science and Technology have pioneered a novel type of palladium hydride nanoparticle, reshaping our understanding of materials that hold immense potential for hydrogen storage and catalytic applications. Palladium, a rare and precious metal, closely resembles platinum and is already celebrated for its catalytic properties.
Published Research
Published in the esteemed Journal of the American Chemical Society, this breakthrough could pave the way for significant advancements in both practical uses and foundational research. Lead investigator Xiao Su, a prominent researcher and professor of chemical and biomolecular engineering at the University of Illinois Urbana-Champaign, emphasized the implications of creating new material phases, stating, "Fundamentally, we have started understanding how electrochemistry can affect the way these unusual nanoparticles are formed."
New Structural Findings
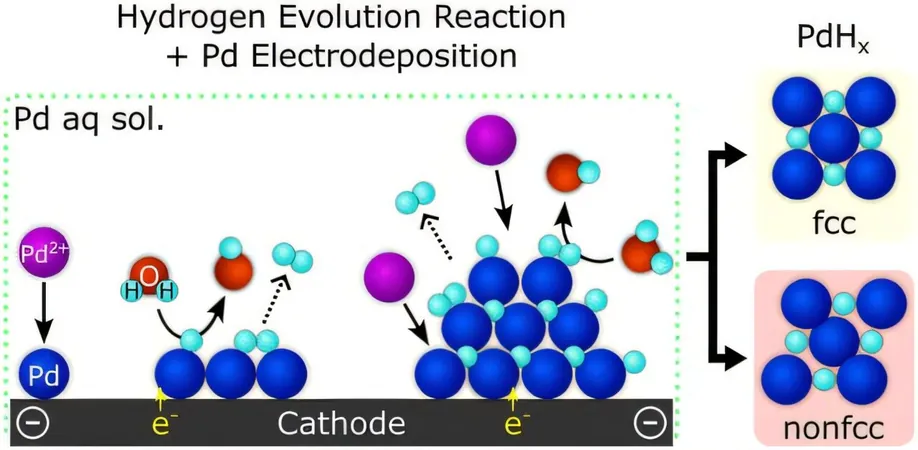
Traditionally, palladium hydride nanoparticles exhibit a symmetrical, cubic structure, with palladium atoms positioned at each corner and at the center of all six faces. However, the newly synthesized nanoparticles possess a remarkable triclinic structure, noted for being one of the least symmetrical of all crystal systems—potentially granting them extraordinary properties.
Innovative Methodology
The innovative approach employed by researchers involves the addition of electrons to a solution containing palladium ions and water, which encourages positive hydrogen atoms from the water to detach and bond with the palladium. This intriguing method contrasts sharply with prior research that relied on a microscopic electron beam to induce a process known as radiolysis for water molecule splitting.
Quotes from Researchers
“This novel technique opens up exciting avenues for the creation of novel materials,” remarked Jaeyoung Hong, the lead author and a dedicated graduate student in Su's lab. He further highlighted the nanoparticles' remarkable ability to retain hydrogen atoms even in a hydrogen-deficient environment, a challenge that conventional palladium hydride faces when in contact with air.
Potential Applications
The potential applications for these groundbreaking nanoparticles are vast. From enhanced hydrogen storage solutions to innovative catalysis in energy conversions, the findings could inspire new pathways for developing metal-nonmetal compound materials.
Conclusion
Stay tuned as these pioneering discoveries continue to transform our understanding of electrochemistry and materials science! Will we soon witness a revolution in energy storage and catalysis thanks to these extraordinary palladium hydride nanoparticles? Only time will tell, but the future looks electrifying!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)