Breakthrough in Fibrosis Treatment: Scientists Identify Key Target for Future Drug Therapies!
2024-09-23
Medical researchers have made a significant stride in the long-standing battle against fibrosis, a condition characterized by excessive scarring of organs that can lead to severe impairment and irreversible loss of function. This study reveals a promising target for drug therapy, which could revolutionize treatment options for patients suffering from various fibrotic diseases.
Fibrosis occurs when abnormal connective tissue builds up in organs, adversely affecting their ability to function correctly. This condition often arises during chronic inflammation, which can be triggered by persistent infections, chemical exposures, or extensive tissue injuries. In many cases, patients find it challenging to identify the underlying cause of their fibrosis, leaving them with few treatment options, and in severe instances, it can lead to fatal outcomes.
Notably, fibrosis can affect major organs like the lungs—causing idiopathic pulmonary fibrosis—and the liver, resulting in conditions like fatty liver disease. Cardiac and kidney fibrosis are other serious manifestations, where scarring, notably after events like heart attacks, leads to myocardial fibrosis. Unfortunately, current therapies lack the ability to effectively treat or reverse these damaging conditions, thereby making the search for novel therapeutic strategies a critical research priority.
Recent findings from medical researchers at the Westmead Institute for Medical Research in Sydney, Australia, shed light on the mechanisms sustaining fibrosis. Led by Dr. Ziyan Pan, the researchers focused on understanding the feedback loop that perpetuates fibrotic scarring. Their groundbreaking study, published in Science Translational Medicine, emphasizes the crucial role of a specific molecular activity in this process.
The researchers observed that while transforming growth factor-beta (TGF-β) is known to regulate fibrosis, it is also involved in various essential biological processes, complicating its viability as a drug target. Dr. Pan articulated the desire to discover alternative methods to inhibit TGF-β signaling, using human and mouse cell models for their investigations.
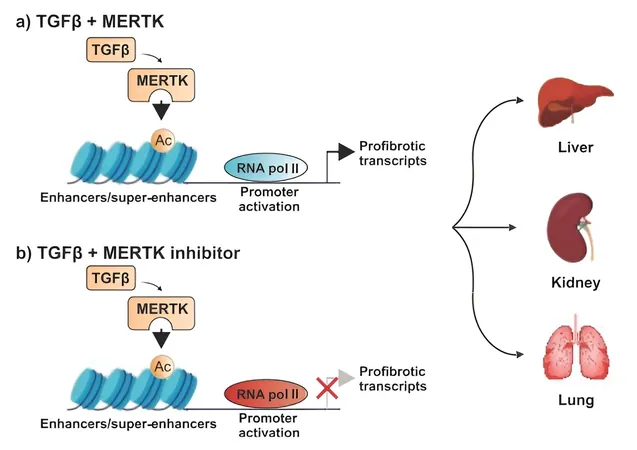
A critical discovery in their research was the identification of elevated levels of an enzyme known as MERTK in various organs affected by fibrosis. This was corroborated by liver biopsy samples from patients with advanced stages of fatty liver disease, demonstrating heightened MERTK levels.
By experimenting with mouse models, the scientists revealed that MERTK does not only foster the expression of TGF-β but also drives its signaling, establishing a positive feedback loop that exacerbates fibrosis. Remarkably, a MERTK inhibitor named UNC569 was introduced, leading to significant reductions in fibrosis across the liver, kidneys, and lungs when administered early in the disease process. Encouragingly, this experimental drug also showed the ability to reverse established liver fibrosis.
Dr. Pan highlighted, “Our findings suggest that inhibiting MERTK can disrupt the fibrosis-promoting signaling loop, offering a beacon of hope for treating fibrotic diseases.”
While further studies are needed to transition this research into human clinical trials, the implications are profound. The identification of MERTK as a pharmaceutical target opens a pathway toward developing more effective treatments across a range of fibrotic conditions. This research not only enhances the understanding of MERTK’s role in fibrosis but also uncovers potential regulatory molecules that can impact TGF-β activity during the disease process.
As hope emerges for innovative treatments, the battle against fibrosis takes a critical step forward, shining a light on the possibility of reversing the effects of this debilitating condition. Keep an eye on this exciting research as we await further developments!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)