Breakthrough in Lysosome Transport: Scientists Unlock Secrets Behind Key Protein Complex
2025-01-14
Author: Ming
Introduction
In a groundbreaking study, researchers led by Professor Feng Wei at the Institute of Biophysics, Chinese Academy of Sciences, have unveiled the intricate workings of the BORC complex, a critical player in lysosome transport and localization. This achievement promises to deepen our understanding of cellular transport mechanisms and their implications for health and disease.
The Role of the BORC Complex
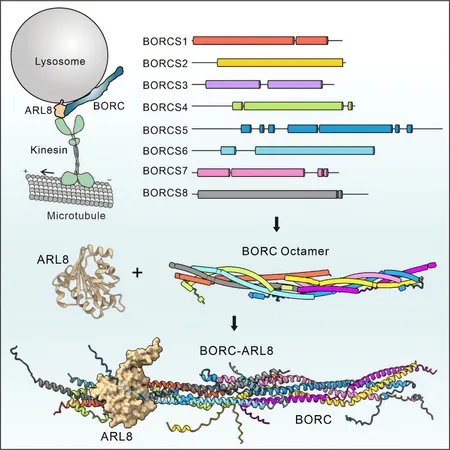
The BORC complex, an assembly of eight proteins, plays a vital role in recruiting the small G protein ARL8, which mediates the transport and proper positioning of lysosomes within the cell. This dynamic duo is an essential regulatory component in the fusion of lysosomes with autophagosomes and the targeting of newly synthesized proteins to lysosomes — processes that are crucial for cell function and maintaining cellular homeostasis.
Major Breakthrough in Understanding Molecular Mechanism
Despite its significance, the exact molecular mechanism by which the BORC complex forms and interacts with ARL8 remained shrouded in mystery until now. Feng and his team have made a major breakthrough by revealing the coiled-coil structure of the BORC complex, identifying key regions that facilitate its interaction with ARL8. Their findings, published in the journal Structure on December 30, 2024, lay the groundwork for further exploration into lysosomal transport mechanisms.
Methodology: Advanced Techniques in Action
Employing advanced cryo-electron microscopy (cryo-EM), the researchers discovered that the BORC complex deviates from the expected "bead-on-a-string" structure, instead showcasing a more complex coiled-coil arrangement. This discovery was no small feat, as structural biology often struggles with such intricate assemblies.
To tackle the technical hurdles associated with cryo-EM samples, the team utilized crosslinking mass spectrometry and cutting-edge AlphaFold structural prediction methods, enabling them to accurately map the BORC complex's structure. Their analysis revealed that the octameric BORC complex forms an elongated rod-like structure composed of two hemicomplexes. Each hemicomplex consists of four distinct subunits, organized into tightly packed coiled-coil bundles.
New Insights on Sub-Complexes
Furthermore, the study unveiled that BORC can sequentially recruit additional subunits through its core sub-complex, indicating the presence of several biologically significant sub-complexes operating within living cells. In a riveting twist, the researchers discovered that the interaction between BORC and ARL8 depends on a specific peptide sequence at the N-terminus of the BORCS5 subunit. Validation of this interaction was demonstrated through the creation of a BORCS5 knockout cell line using CRISPR/Cas9 technology, confirming its critical role in lysosome transport.
Broader Implications and Future Directions
Professor Feng highlighted the broader implications of this research, stating, "Lysosomal dynamic transport is essential for the growth and invasion of cancer cells and represents a potential target for anti-cancer therapies. Our study provides important theoretical support for the biological study of lysosome transport and localization."
As scientists continue to unravel the complexities of cellular transport, this research not only offers hope for advancing our understanding of cellular processes but also opens up innovative avenues for therapeutic interventions in cancer and other diseases. The race to explore the potential of targeting lysosomal transport could very well revolutionize the treatment landscape in oncology.
Conclusion
Keep watching this space for more exciting developments in the world of cellular biology!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)