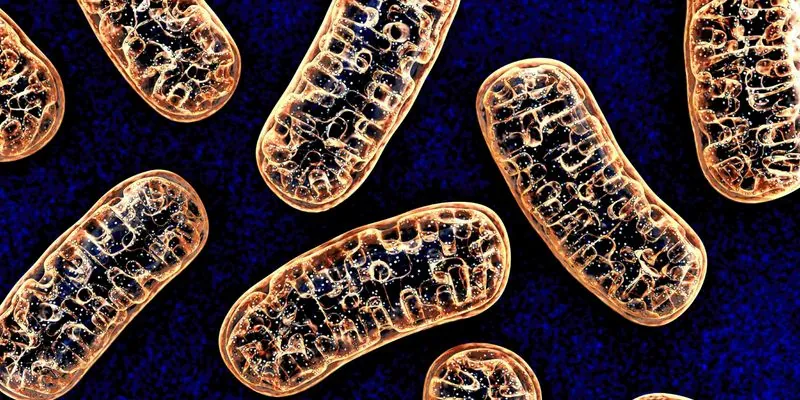
Breakthrough in Mitochondrial Disease Treatment: New Drug Shows Unprecedented Promise!
2025-04-09
Author: Daniel
Introduction
In a groundbreaking step toward treating mitochondrial diseases, new research reveals that a small molecule drug called sonlicromanol may significantly improve the health of patients suffering from a specific genetic mutation known as m.3243A>G. Mitochondrial diseases, usually marked by debilitating symptoms due to failures in energy production within cells, have long been considered challenging to manage, with no effective treatments available until now.
Understanding Mitochondrial Diseases
Mitochondrial DNA mutations can lead to hazardous imbalances between mutant and healthy mitochondria within cells, a situation referred to as heteroplasmy. As this balance tips, symptoms of mitochondrial disease manifest in various forms, unique to each patient and even amongst family members. This variability makes it incredibly difficult for researchers and physicians to develop universally effective treatments.
The Mission of Jan Smeitink
Recognizing the urgent need for progress in this field, Jan Smeitink, a pediatrician and mitochondrial biologist at Radboud University, embarked on a mission to create viable therapeutic options. In 2007, he founded Khondrion, a company dedicated to pioneering treatments for mitochondrial disorders, where he now holds the position of CEO.
Sonlicromanol and Phase 2b Trial Results
Recent findings published in the journal Brain detail the results of a Phase 2b trial for sonlicromanol, specifically targeting individuals with the troublesome m.3243A>G mutation in the mitochondrial gene MT-TL1. Participants who received the drug reported substantial health improvements, and the treatment was found to be safe and well-tolerated.
Impact of the m.3243A>G Mutation
The m.3243A>G mutation, the most prevalent variant associated with mitochondrial diseases, disrupts the cellular machinery responsible for producing vital proteins necessary for energy generation. Patients with this mutation often face a spectrum of debilitating symptoms, such as hearing loss and stroke-like episodes, leading to significant declines in quality of life.
Mechanism of Action of Sonlicromanol
Sonlicromanol is an innovative oral medication designed to penetrate the brain. It functions by enhancing ATP, the energy currency of the cell, while simultaneously mitigating oxidative stress and inflammation that contribute to cellular damage and death.
Study Design and Findings
During the study, a randomized controlled trial was conducted to ascertain the drug's optimal dosage. Following this, a 52-week open-label extension allowed researchers to evaluate the long-term effects and safety of sonlicromanol. The outcomes far exceeded initial expectations.
Patient Improvements and Observations
Smeitink expressed his astonishment: “That, I’ve never seen before. I’ve seen thousands of patients with mitochondrial disease, and they always get worse over time. But after treatment with sonlicromanol, their conditions improved!” Patients demonstrated enhancements in various areas, including quality of life, mood, fatigue levels, and muscle function. Notably, the Newcastle Mitochondrial Disease Adult Scale (NMDAS), used to gauge disease severity, indicated a downward trend in scores after treatment—an unprecedented finding in the history of this condition.
Cognitive Function and Variability
Herma Renkema, Chief Scientific Officer at Khondrion and a collaborator on the study, noted that some cognitive functions did not show significant improvement—a fact attributed to participant variability in blindness scores. Nonetheless, when scores were adjusted, sonlicromanol showed marked advantages over placebo groups.
Challenges in Treatment
The complexity of treating such heterogeneous patient populations is well understood. Mike Murphy, a biochemist from the University of Cambridge who was not involved with the study, remarked on the challenges of conducting trials amidst significant variability in symptoms and genetic backgrounds. Regardless of these obstacles, he praised Khondrion’s commitment to finding solutions for those afflicted by mitochondrial disease.
Looking Ahead to Phase 3 Trials
Looking ahead, Smeitink and his team are eager to initiate a Phase 3 clinical trial to further investigate sonlicromanol’s potential. After receiving FDA clearance for their Investigational New Drug (IND) application in November, they are gearing up to enroll 150 adult patients bearing the troublesome m.3243A>G mutation.
Conclusion and Future Prospects
"We expect to have the first patients in early in the second half of this year," Smeitink said. “The sooner, the better.” The anticipation surrounding this groundbreaking research underscores the hope for effective treatments that could transform the lives of those living with mitochondrial disease. Keep your eyes peeled, as this promising journey may pave the way for new horizons in mitochondrial medicine!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)