Breakthrough in Protein Synthesis: New Firefly Luciferase Reporter Unveils Hidden Issues
2024-12-06
Author: Yu
In an exciting development out of Science Tokyo, researchers have unveiled a revolutionary firefly luciferase-based reporter capable of detecting intricate problems during protein synthesis, particularly in the endoplasmic reticulum (ER). This innovative tool may pave the way for significant advancements in understanding various diseases and could ultimately lead to new therapeutic strategies.
Understanding Protein Synthesis and Its Challenges
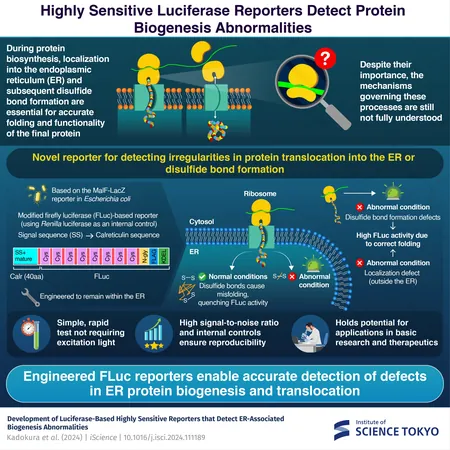
In eukaryotic organisms—such as plants, animals, and fungi—protein synthesis is not merely a straightforward process of amino acid assembly. Surprisingly, nearly a third of all human proteins are required to navigate to the endoplasmic reticulum shortly after their synthesis. Inside the ER, they undergo essential structural modifications, including the formation of disulfide (S-S) bonds, crucial for proper protein function.
Disruptions in either protein translocation to the ER or disulfide bond creation can lead to serious health issues, including neurodegenerative diseases and various forms of cancer. Despite the significance of these processes, traditional methods to study them have proven inadequate, often requiring expensive equipment and sophisticated techniques.
The Breakthrough Reporter Molecule
The research team, led by Specially-Appointed Associate Professor Hiroshi Kadokura and Professor Hideki Taguchi, has crafted a groundbreaking reporter molecule that simplifies these studies. Their findings are detailed in the prestigious journal *iScience*.
Taking inspiration from the MalF-LacZ fusion protein in *Escherichia coli*, which identifies issues in protein transport, the researchers engineered a luciferase variant that accurately reflects disruptions in protein synthesis within the ER. The luciferase enzyme emits light by catalyzing the oxidation of D-luciferin, but the team modified it to become inactive upon proper disulfide bond formation inside the ER, while remaining active in the cytosol or unmodified.
Implications and Applications
Through this clever design, any failure in transporting proteins or forming disulfide bonds would result in an unexpectedly active luciferase signal, alerting researchers to problems in the process.
This new tool also includes a unique glycosylation motif that changes only upon successful ER translocation, enabling the team to distinguish between translocation issues and disulfide bonding defects. This dual functionality provides a comprehensive view of protein synthesis challenges.
The team's experiments showcased the versatility of this reporter, demonstrating its sensitivity in cells where the ER's redox state was chemically altered, impeding disulfide bond creation. They also utilized it to identify defects in protein transport related to a potential anti-HIV drug, indicating its relevance in antiviral research.
Kadokura emphasized the assay's high throughput capability, suggesting that this approach could revolutionize the large-scale screening of compounds that inhibit the production of harmful proteins involved in the secretory pathway.
Advantages of the New Reporter System
This luciferase-based reporter holds several advantages, including straightforward use, resilience to environmental changes, and high reproducibility. As Taguchi noted, 'Our system is set to become an indispensable tool across various disciplines related to secretory proteins, extending far beyond basic research.'
As this technology evolves, it may unlock new avenues in disease understanding and treatment development, solidifying its impact on both basic and applied sciences. Don't miss out on the potential breakthroughs that could stem from this innovative research!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)