Breakthrough Protocol Revolutionizes Cervical Cancer Screening for Women!
2024-12-13
Author: Daniel
Introduction
A groundbreaking new protocol for analyzing self-collected cervical samples promises to significantly cut back the number of women who receive unnecessary recalls for clinician-collected cervical screenings. This innovative approach not only streamlines the screening process but may also expedite referrals for necessary gynecological assessments, enhancing the effectiveness of cervical cancer screening in the NHS.
Research Overview
Led by researchers at Queen Mary University, this study utilized an extensive dataset related to self-collected samples to assess the risk of significant disease presence among women. The results, published in the renowned journal PLOS Medicine, indicate that this protocol could help minimize unnecessary clinical appointments while allowing a more rapid transition for those in need of specialized care.
Cervical Cancer Screening Trends
Cervical cancer, which is largely preventable, is currently facing a disturbing trend—the participation in the NHS Cervical Screening Program is plummeting to record lows. One solution that has gained traction is the use of self-sampling HPV (human papillomavirus) tests, where women can collect their samples from the comfort of their own homes. Remarkably, recent research shows that nearly 70% of women prefer self-sampling over in-clinic visits.
NHS Considerations
In a notable move, the UK National Screening Committee is currently deliberating whether to incorporate the option of HPV self-sampling into the NHS cervical cancer screening program, making screening more accessible to those who have been under-screened.
Challenges of Self-Sampling
While clinician-collected samples are routinely gathered during GP visits, self-samples present unique challenges, as they cannot be assessed using traditional cytology methods. If a self-sample tests positive for HPV, patients are typically required to undergo a second clinician-collected sample to ascertain the next steps: immediate colposcopy, re-testing in a year, or clearance from the virus. Alarmingly, about 20% of women who test positive on a self-sample do not attend for follow-up clinician sampling, which could result in untreated pre-cancer conditions.
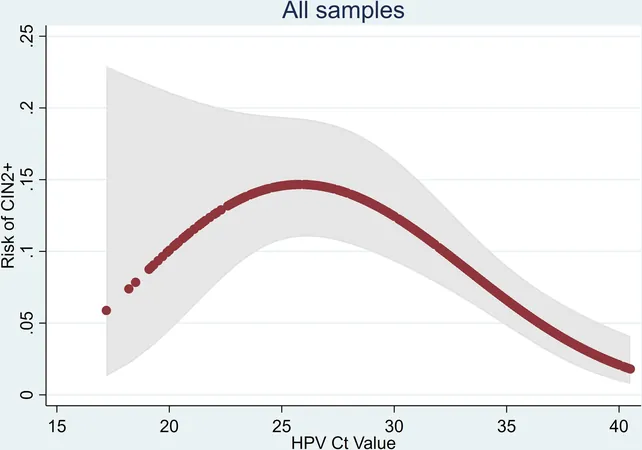
Study Findings
In this study led by Professor Peter Sasieni and Dr. Jiayao Lei from the Karolinska Institute in Sweden, researchers categorized HPV-positive women into three distinctive risk groups: high-risk, medium-risk, and low-risk. The findings revealed that only 5% of participants fell into the high-risk category, yet an impressive 40% of those women had treatable disease, qualifying them for immediate colposcopy. Conversely, a majority—53%—were classified as low-risk, with merely 4% demonstrating high-grade disease, suggesting they could be safely re-tested after 12 months without immediate clinician intervention.
Expert Commentary
Professor Sasieni, an esteemed cancer epidemiologist, stated: "Our analysis indicates that the majority of women who initially test positive from self-samples could be re-tested after a year instead of undergoing another in-clinic screening. This innovative risk assessment could not only reduce the number of unwarranted recalls but also ensure that we capture a significant proportion of cervical cancer cases in their early stages."
Conclusion
This transformative protocol may represent a turning point in cervical cancer prevention, providing women with safer, more acceptable options as necessary while working to address the concerning decline in screening participation. As technologic advances continue to shape healthcare, the future of cervical cancer screening looks promising. What does this mean for women's health moving forward? Stay tuned as we follow this developing story!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)