Breakthrough Study Reveals Secrets Behind Atmospheric Chemistry: Are Criegee Intermediates the Key to Understanding Air Quality?
2024-11-25
Author: John Tan
A groundbreaking study has shed light on the formation of Criegee intermediates (CI) and organic peroxy radicals (RO2), two critical components in atmospheric chemistry that significantly affect air quality and climate change. These reactive species are essential in the process that leads to the formation of secondary organic aerosols (SOA), which play a vital role in determining the earth's atmospheric conditions.
Conducted by a dedicated team from the Institute of Earth Environment at the Chinese Academy of Sciences, this research dives deep into the mechanisms of how Criegee intermediates are produced through the oxidation of common alkenes like ethylene (C2H4), propylene (C3H6), and 2-methylpropene (2-CH3-C3H5). By applying advanced quantum chemical and kinetic modeling techniques, the study has unveiled new insights that were recently published in the journal Atmospheric Environment.
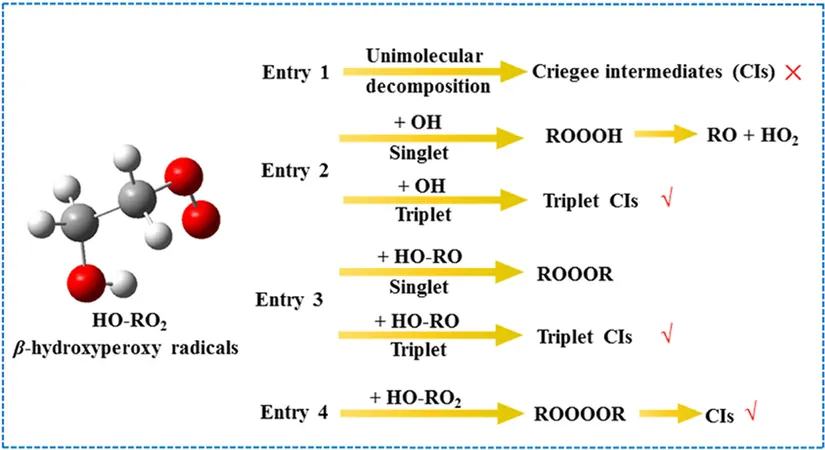
The researchers discovered that the main reaction pathway for the interaction of hydroxyl-initiated organic peroxy radicals (HO-RO2) involves a barrierless reaction that leads to the formation of a compound known as ROOOH. This intermediary is key because its breakdown into RO and HO2 radicals represents the most energetically favorable route. Intriguingly, they noted that the presence of additional methyl groups increases the stability of ROOOH, indicating the potential influence of molecular structure on chemical behavior.
Further investigations revealed that in the case of reactions involving HO-RO2 radicals, a similar barrierless formation occurs with a compound referred to as ROOOR. Here, separating back to the original reactants is also a preferred pathway, showing that the intricacies of molecular arrangements can affect stability and reaction pathways.
Excitingly, the study points out that as the number of methyl substituents increases, the barriers and energy requirements for forming carbonyl oxides from self-interaction of HO-RO2 radicals decrease. This means that structural variations play a crucial role in atmospheric reactions, potentially impacting how pollutants interact in the environment.
Overall, this study not only enhances our understanding of the traditional pathways associated with peroxy radicals and Criegee intermediates but also holds significant implications for improving the accuracy of numerical models used to predict air quality. As human activities continue to emit pollutants into the atmosphere, understanding these mechanisms becomes ever more critical in our fight against climate change and its myriad effects on our health and environment.
Could this research be the turning point in how we combat air pollution? Only time will tell!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)