Bromoform's Dance with Light: A Stunning Discovery Unveils Molecular Secrets
2024-11-19
Author: Arjun
Introduction
In a groundbreaking study, scientists have unveiled new insights into the behavior of bromoform (CHBr3), a natural compound notorious for its ozone-depleting properties in Earth's atmosphere. This research has revealed that when exposed to ultraviolet (UV) light, bromoform molecules embark on a fascinating molecular dance, with significant implications for our understanding of atmospheric chemistry.
The Molecular Dynamics
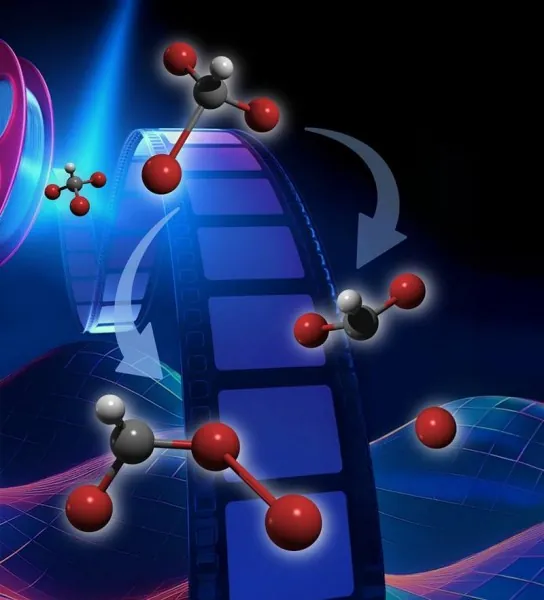
When bromoform interacts with light, it can go through two key processes: "bond dissociation," where one bromine atom detaches from the main structure, and "isomerization," which involves a rearrangement of atoms while keeping the molecule intact. Despite previous research efforts, the complexities of these swift reactions remained an enigma—until now.
In a novel experiment conducted with the cutting-edge relativistic ultrafast electron diffraction (UED) instrument at the SLAC National Accelerator Laboratory, researchers discovered that roughly **60% of bromoform molecules** exposed to UV light engage in isomerization rather than simply breaking apart. This revelation marks a pivotal moment in understanding how these molecules interact with light, suggesting that isomerization plays a more significant role than previously thought.
Confronting Established Theories
The findings of this research challenge prior theoretical frameworks, which had provided some insights into these reactions but often fell short of capturing the full picture. Researchers found that while their results aligned with certain predictions, they defied others. They demonstrated for the first time that isomers formed immediately after the initial exposure to light—specifically within the first fifth of a picosecond—and persisted throughout the duration of the experiment, which lasted 1.1 picoseconds. These observations push the boundaries of current theories on bromoform's behavior, signaling a need for refined theoretical modeling.
Environmental Implications and Future Directions
The implications of this research extend beyond mere molecular curiosity. Bromoform's ability to deplete ozone makes understanding its reactions in the atmosphere crucial for environmental science and policy-making. By elucidating the mechanisms behind its interaction with UV light, scientists can better predict its atmospheric behavior and, consequently, its impact on climate change.
The crucible of scientific inquiry into bromoform has set the stage for future explorations. The precise ratio of isomerization to bond dissociation not only opens the door to a deeper understanding of bromoform's chemistry but also highlights the intricate dance of molecular interactions that are quickly becoming central to various fields of study—from atmospheric science to materials engineering.
Conclusion
This pioneering research on bromoform molecules showcases the remarkable advances in molecular spectroscopy and chemistry. As scientists continue to explore the intricacies of these rapid processes, they uncover layers that could reshape our comprehension of environmental chemistry and molecular behavior. The dance of bromoform in the light not only enriches scientific knowledge but could ultimately play a role in addressing some of the pressing challenges related to climate change and atmospheric health. Stay tuned as further discoveries unfold!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)