Discover the Promise of Zinc-Dependent Proteins in Fighting Cancer and Viral Infections!
2024-12-17
Author: Wei
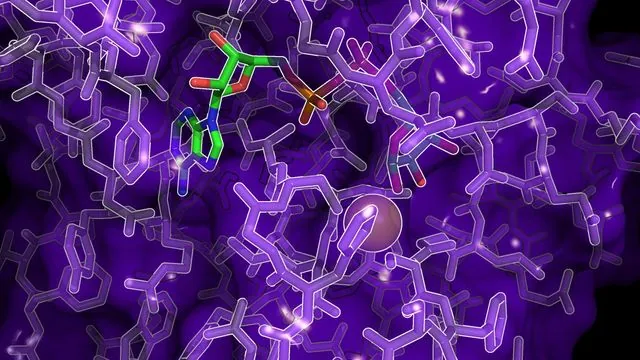
In an exciting advancement for medical science, researchers at the University of Sheffield, UK, have revealed groundbreaking insights into zinc-dependent proteins, particularly macrodomains, which could reshape the way we approach cancer and viral therapies. Published recently in The Journal of Biological Chemistry, this comprehensive study unveils how these proteins are pivotal in reversing a critical cellular modification known as ADP-ribosylation, a process often exploited by cancer cells and viruses to thrive.
Why This Research Matters
As the medical community grapples with cancer's increasing resistance to standard therapies, there is a pressing need to understand the molecular mechanisms behind disease progression. Cancer cells, along with various pathogens, have adapted to utilize cellular processes to boost their survival rates. Understanding the interaction between zinc-dependent macrodomains and ADP-ribosylation is crucial, as these proteins play a significant role in cellular stress responses, and hijacking this process offers an avenue for new therapeutic targets.
Decoding the Molecular Mechanism
The researchers employed sophisticated techniques like X-ray crystallography to map how zinc ions facilitate the hydrolysis of ADP-ribose—essentially reversing the harmful effects of ADP-ribosylation. This research confirms that zinc is not merely a secondary element but a critical player at the active site of these macrodomains. The implications are profound, as these insights could lead to innovations in drug development aimed at disrupting the adaptive mechanisms of diseases.
The Potential for Targeted Drug Development
One of the standout revelations of this study is that zinc-dependent macrodomains, already recognized for their high conservation across various species, are plentiful in pathogens. This makes them prime candidates for new antimicrobial strategies, particularly as antibiotic resistance grows more concerning. By targeting these proteins, researchers could develop treatments that not only enhance our arsenal against infections, particularly those caused by bacteria and fungi, but also tackle cancer more effectively.
But Wait, There’s More!
The significance of this research extends beyond ADP-ribosylation's importance in cellular resilience. The findings indicate that the same mechanisms hijacked by pathogens and cancer cells can become the Achilles' heel of these diseases when properly targeted. Envision therapies that specifically inhibit the dysfunctional ADP-ribosylation processes in tumors or viral infections—this could represent a paradigm shift in how we treat currently intractable diseases.
A Note of Caution
Despite the promising nature of these findings, there are important considerations to keep in mind. The controlled laboratory environments used in these studies may not fully replicate the highly complex interactions occurring in living organisms. As a result, further research in varied biological models is essential to validate these discoveries and ensure that potential therapies will be effective in real-world applications.
What’s Next on the Horizon?
Looking ahead, future investigations will focus on the in-depth exploration of zinc-dependent macrodomains across different disease models. Understanding how these macrodomains operate within the broader context of cellular environments could unveil additional molecular factors influencing ADP-ribosylation, ultimately enhancing the viability of these targets in drug discovery.
In conclusion, the unearthing of zinc-dependent macrodomains as crucial players in regulating ADP-ribosylation offers a glimpse of hope in the fight against diseases that have long evaded effective treatment. This research not only paves the way for novel therapeutic strategies but also emphasizes the increasing importance of understanding the intricate molecular dance within our cells. Stay tuned as this groundbreaking journey unfolds, potentially transforming the landscape of cancer and viral therapies!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)