Discovering the Genetic Factors Behind COVID-19 Severity: How PAI-1 Polymorphisms Could Hold the Key
2024-10-02
Author: Rajesh
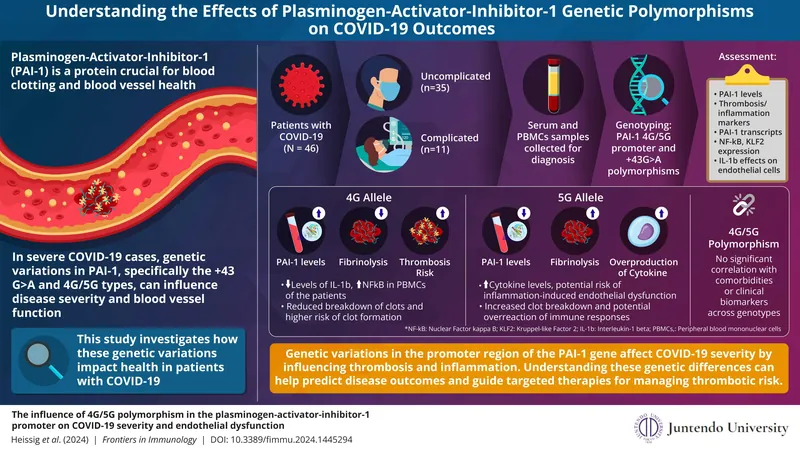
Despite worldwide vaccination efforts, COVID-19 remains a potent threat, resulting in serious complications and high mortality rates. Critical to understanding these outcomes is the role of the coagulation and fibrinolytic systems—those that either promote or prevent blood clot formation. Impairments in these systems can lead to heightened inflammatory responses and thrombosis, making it essential to identify the genetic underpinnings that might predispose individuals to severe disease.
At the heart of this understanding lies the plasminogen activator inhibitor-1 (PAI-1) gene, particularly its promoter region's polymorphisms. The most notable of these is the 4G/5G polymorphism, whose implications have seen a surge in research due to its connections to various cardiovascular conditions. A compelling study led by researchers from Juntendo University in Japan sheds new light on how this genetic variation could influence COVID-19 severity.
Published in Frontiers in Immunology on August 30, 2024, researchers evaluated Japanese patients with COVID-19, focusing on the impact of the 4G/5G polymorphism on their thrombotic responses. The team, featuring experts like Dr. Beate Heissig and Dr. Koichi Hattori, collected blood samples to analyze DNA for the polymorphism's presence, correlating it with disease severity as categorized by hospitalization needs and ICU admissions.
The investigation revealed that the two alleles—4G and 5G—promote significantly different biological responses. The 4G allele contributed to a state of hypofibrinolysis, marked by elevated PAI-1 levels that inhibit the breakdown of clots, consequently raising the risk for thrombosis. In line with this, patients showing this allele displayed lower levels of IL-1β, a pro-inflammatory cytokine, but elevated activity of NFκB, a protein complex involved in cellular responses to inflammation.
Meanwhile, the 5G allele demonstrated a contrasting effect, promoting increased fibrinolysis and potent cytokine responses—a scenario with higher chances of successful clot resolution but potentially at the cost of enhanced inflammation. Interestingly, while the study delved into how comorbid conditions like obesity or hypertension might modify PAI-1 expression, it did not find a direct influence of these on the polymorphisms under review.
This groundbreaking study emphasizes the importance of genetic predispositions in determining COVID-19 outcomes. The identification of patients who carry the 4G allele could signal those at elevated risk for severe disease, prompting proactive management strategies. These could involve specific anti-thrombotic or anti-inflammatory therapies aimed at mitigating the risk of complications such as thrombosis and excessive inflammatory responses—often referred to as cytokine storms.
As researchers continue to explore the genetic intricacies related to COVID-19, findings such as these could revolutionize how medical professionals approach treatment. Understanding the 4G/5G polymorphism not only opens a pathway for personalized medicine in the context of COVID-19 but also holds promise for other diseases where inflammation and clot management are critical.
In summary, the connection between PAI-1 polymorphisms and COVID-19 severity stresses the necessity of integrating genetic screening into clinical practices. This approach could significantly enhance patient outcomes and alleviate the broader impact of COVID-19 on public health. Future investigations may even pave the way for therapeutic strategies targeting PAI-1 itself, potentially transforming the landscape of treatment for critically affected patients.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)