Eli Lilly's Breakthrough Drug Shows Remarkable Heart Health Benefits for Obese Patients
2024-11-18
Author: Ming
Eli Lilly's Breakthrough Drug Shows Remarkable Heart Health Benefits for Obese Patients
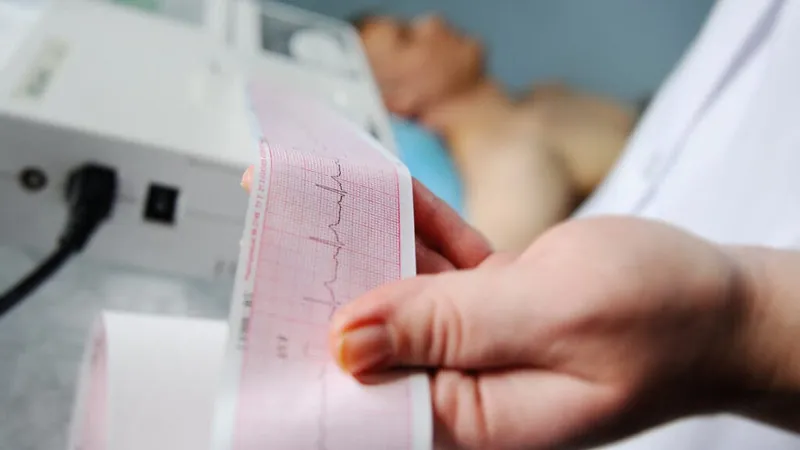
In a groundbreaking development, Eli Lilly and Company (NYSE: LLY) has unveiled the impressive results of its SUMMIT Phase 3 trial, showcasing the exciting potential of tirzepatide, marketed as Mounjaro and Zepbound. This innovative treatment has demonstrated significant cardiovascular benefits for adults suffering from heart failure with preserved ejection fraction (HFpEF) in conjunction with obesity.
The Phase 3 trial involved 731 participants and revealed that those treated with tirzepatide experienced a remarkable 38% reduction in the risk of cardiovascular mortality and worsening heart failure events over an average follow-up period of nearly two years. This translates to a reduction of cardiovascular-related events from 15.3% in the placebo group to an impressive 9.9% among those receiving tirzepatide.
But the benefits don’t stop there. Patients on tirzepatide reported substantial improvements in their quality of life, showcasing nearly a 25-point increase on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), which evaluates heart failure symptoms and limitations. Comparatively, the control group only experienced a 15-point uplift in this crucial measure.
Diving deeper into the trial's findings, tirzepatide participants exhibited a 56% reduction in heart failure-related hospitalizations compared to their placebo counterparts. Furthermore, participants on the medication walked an astonishing 30 meters further in a six-minute exercise test than those receiving placebo, a clear indicator of enhanced physical capacity.
Tirzepatide isn't just helping patients feel better; it’s also contributing to significant weight loss. Participants reported an average weight reduction of 15.7%, which can further relieve stress on the heart. Conversely, the placebo group only saw a minimal 2.2% loss in weight.
Notably, the drug also targets inflammation, a key factor in cardiovascular disease. Tirzepatide led to a 43.4% decrease in high-sensitivity C-reactive protein levels, a substantial drop compared to the meager 3.5% reduction observed in the placebo group.
The U.S. Food and Drug Administration (FDA) previously approved tirzepatide in May 2022 as Mounjaro for adults with type 2 diabetes. Recently, in November 2023, it was further approved as Zepbound for individuals facing obesity or overweight issues accompanied by at least one comorbidity related to their weight. With the promising SUMMIT trial results, Eli Lilly has already begun submitting this compelling data to the FDA and European Medicines Agency to widen the drug's labeling for HFpEF and obesity treatments.
As the tide turns in the battle against obesity-related heart conditions, tirzepatide could undoubtedly pave the way for a healthier future for countless patients, combining weight loss and improved heart health in one powerful treatment. This is certainly a development worth keeping an eye on! Stay tuned for more updates and breakthroughs in cardiovascular healthcare!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)