Groundbreaking Discovery: Toxic Protein May Shed Light on ALS Development!
2024-10-14
Author: Wei Ling
A recent study from the Penn State College of Medicine reveals startling insights into amyotrophic lateral sclerosis (ALS), a devastating neurodegenerative disease. Researchers have identified that a toxic variant of a well-known protein can impact brain, spinal cord, and skeletal muscle tissues in distinct ways, potentially driving the complexities of ALS progression.
This pivotal research has made strides in elucidating the physiological mechanisms that contribute to ALS, presenting a possible therapeutic avenue for future interventions. The findings were published in the esteemed journal Structure.
The Focus of Investigation
The focus of this investigation centers around superoxide dismutase 1 (SOD1), a protein closely associated with ALS, particularly in its trimeric form. While SOD1 generally exists as a dimer, composed of two identical protein units, it can dangerously rearrange into a trimer, posing significant cellular threats. Senior author Dr. Nikolay Dokholyan, a G. Thomas Passananti Professor at Penn State, urges the scientific community to explore how these SOD1 trimers inflict cellular harm.
Understanding ALS
ALS is characterized by the progressive degeneration of motor neurons in the central nervous system, leading to severe muscle weakness and wasting. Genetic mutations in SOD1 are implicated in up to 20% of known ALS cases. Prior studies have suggested that SOD1 trimers display a new, toxic capacity unlike the harmless dimers, linked with increased neuronal cell death.
Experimental Findings
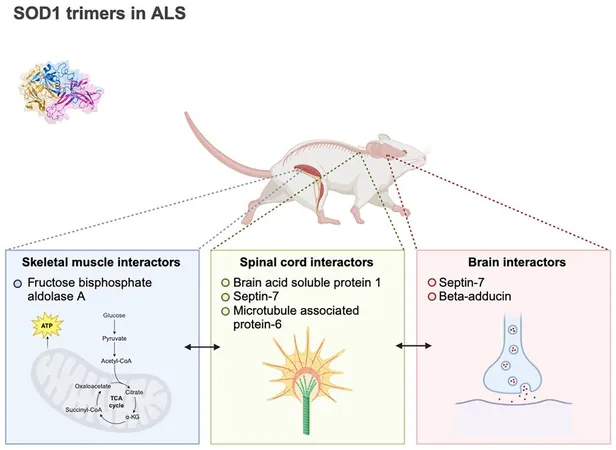
To delve deeper, the researchers conducted experiments involving various mouse tissues—brain, spinal cord, and muscle—to uncover which proteins associate with SOD1 trimers. Co-author Brianna Hnath, a doctoral candidate in biomedical engineering, emphasized their objective: "We were searching for new protein interactions that could reveal how this SOD1 trimer might induce toxicity."
Their findings were surprising; SOD1 trimers interacted with different proteins based on the type of tissue examined. In brain and spinal cord tissues, these trimers engaged with proteins essential for neuronal structure and communication, potentially disrupting neuronal functions and accelerating cellular aging processes. Meanwhile, in muscle tissue, the interactions appeared to intervene directly in metabolic pathways, jeopardizing energy production in muscle cells.
Significance of the Discovery
Hnath expressed the significance of their discovery: "Finding varying interactions across different tissues suggests that distinct mechanisms may contribute to the dysfunction and death of cells based on their type." This challenges the prevalent view that muscle atrophy in ALS solely results from motor neuron degeneration. The study implies that SOD1 trimers may also instigate direct disruptions in muscle cells, exacerbating both muscle and nerve degeneration.
Focus on Septin-7
The researchers spotlighted septin-7, a protein that binds specifically to SOD1 trimers but not to its native dimers. Septin-7 is crucial for maintaining neuronal structure and communication, both vital processes that could be impaired through SOD1 interaction, leading to neuron degeneration. This raises an intriguing prospect: could targeting the interaction between SOD1 trimers and septin-7 hold therapeutic promise in modifying the ALS disease course?
Future Research Directions
As the study points out, further research is essential to clarify SOD1 trimers' role in ALS, understand the underlying mechanisms of cellular dysfunction, and explore the potential of septin-7 as a therapeutic target. The quest to combat ALS continues, with this groundbreaking research illuminating a pathway that could lead to impactful treatments in the future.
Stay tuned for more developments as scientists strive to unravel the mysteries of ALS and work tirelessly toward finding a cure!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)