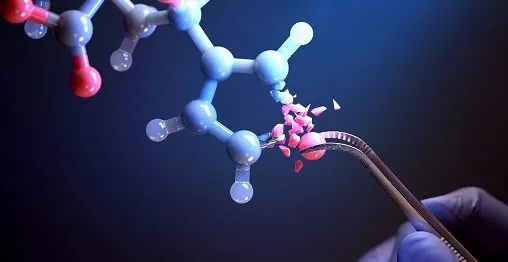
Groundbreaking Light-Catalyzed Technique Revolutionizes Pharmaceutical Chemistry
2024-10-11
Author: Nur
Introduction
Heterocycles, the intricate molecular rings commonly found in numerous pharmaceuticals, have long been notoriously resistant to modification. The challenge has traditionally been that changing one type of heteroatom, like substituting oxygen with nitrogen, is an arduous task that often disheartens medicinal chemists.
Innovative Solution from KAIST
However, researchers at the Korea Advanced Institute of Science and Technology (KAIST) have unveiled an innovative solution that simplifies the single-atom editing process of heterocycles. Their method efficiently swaps an oxygen atom in a furan—a five-membered heterocycle— for a nitrogen atom, resulting in the creation of a pyrrole. This sort of transformation typically demands extreme measures like high-temperature pyrolysis or intense ultraviolet irradiation, but the KAIST team has achieved it under mild conditions, utilizing a photocatalyst activated by low-energy visible light.
Publication of Findings
The details of this pioneering method were recently published in the esteemed journal *Science*, under the title, "Photocatalytic furan-to-pyrrole conversion." The authors highlighted their “photocatalytic strategy that directly converts a furan into a pyrrole analog in a single intermolecular reaction,” demonstrating high compatibility with various furan derivatives and nitrogen nucleophiles, which are frequently utilized in drug development. This advanced technique allows for the late-stage functionalization of complex molecules, facilitating access to challenging-to-synthesize pyrroles derived from naturally occurring furans.
Importance of the Single-Atom Effect
In the realm of drug development, the significance of specific atoms cannot be overstated. Atoms like oxygen and nitrogen are crucial for enhancing drug efficacy, especially against viral infections. This principle, termed the Single-Atom Effect, underscores the dramatic impact that a single atom can have on a drug's performance.
Challenges in Traditional Methods
Traditionally, leveraging this Single-Atom Effect has required complex, multi-step synthesis processes, proving to be both time-consuming and expensive. Yet, the KAIST research team, led by Dr. Yoonsu Park, has overcome these barriers by introducing a state-of-the-art light-activated catalyst that acts like "molecular scissors." This catalyst facilitates the cutting and attachment of five-membered rings at room temperature and atmospheric pressure—an advancement that could transform the landscape of pharmaceutical chemistry.
Mechanism of the Reaction
The team’s innovative reaction mechanism involves the excited molecular scissor, which removes oxygen from furan through single-electron oxidation, followed by the sequential addition of a nitrogen atom. "Mechanistic analysis suggests that polarity inversion through single electron transfer initiates redox-neutral atom exchange processes at room temperature," the researchers stated.
Authors' Insights
First authors Donghyeon Kim and Jaehyun You, both candidates in KAIST's integrated master’s and doctoral chemistry program, emphasized the technique's versatility and its capacity to replace harsh reaction conditions with light energy. This new capability offers the potential for selective edit even in complex natural products or pharmaceuticals.
Conclusion and Future Prospects
"This groundbreaking advancement in selectively editing five-membered organic ring structures will vastly improve the construction of libraries of drug candidates, which has always been a significant challenge in the pharmaceutical industry," Dr. Park declared. He expressed optimism that this foundational technology will revolutionize the drug development process, heralding a new era in medicinal chemistry.
What's Next?
Drug developers and researchers are poised to explore the vast possibilities of this innovative light-catalyzed method, potentially leading to more effective treatments and a faster pathway from laboratory to market. Keep an eye on the future of pharmaceuticals as this technique unfolds new opportunities for creating next-generation drugs!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)