Groundbreaking Method to Synthesize Key Compound for Eco-Friendly Rubber Production
2024-11-23
Author: Li
Introduction
In a remarkable breakthrough, chemists at the National University of Singapore (NUS) have unveiled a pioneering and sustainable method to electrosynthesize 1,3-butadiene, a vital compound in the production of synthetic rubber, directly from acetylene. This innovative approach is poised to transform the synthetic rubber industry and significantly reduce its environmental impact.
Importance of Energy-Efficient Production
Producing multi-carbon molecules like 1,3-butadiene in an energy-efficient manner is essential for fostering a greener and more sustainable chemical industry. Traditional methods often rely on fossil fuels and are energy-intensive, but electrification—a process that utilizes renewable electricity—offers a promising alternative. This technique efficiently converts simple feedstocks such as carbon dioxide (CO2) and water into valuable chemicals and fuels.
Traditional Production Methods
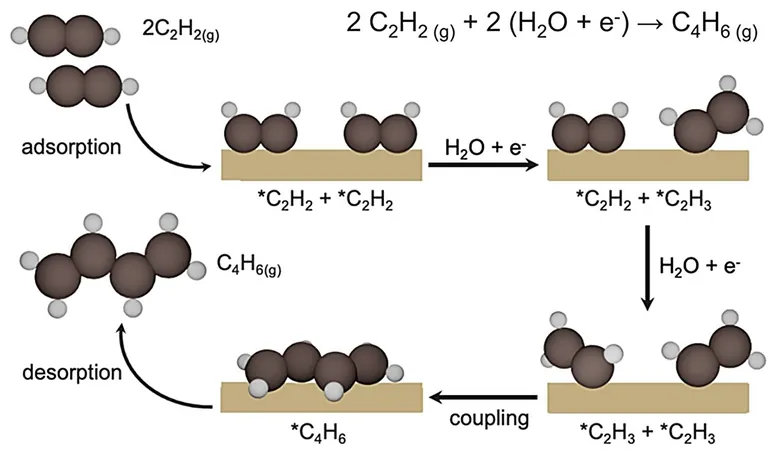
1,3-butadiene itself is predominantly produced as a by-product alongside ethylene through the conventional and energy-intensive cracking of naphtha or ethane, generating over 18 million tons annually. The NUS research team, led by Associate Professor Yeo Boon Siang, Jason, made significant advances by discovering that modified copper catalysts could effectively convert acetylene into 1,3-butadiene with impressive results.
Research Findings
The study published in the esteemed journal *Nature Catalysis* highlights the use of copper catalysts, enhanced through a simple modification with iodide anions. Remarkably, this technique achieved a Faradaic efficiency of 93% at -0.85 V versus the Standard Hydrogen Electrode (SHE), along with an astounding partial current density of -75 mA cm⁻² at -1.0 V. This catalytic activity is at least 20 times greater than previous studies.
Collaborative Efforts
This pioneering research is the result of collaborative efforts involving international experts, including Dr. Federico Calle-Vallejo from Spain's Basque Foundation for Science, alongside contributors from various institutions including Shell Global Solutions International B.V. and the University of Barcelona.
Catalyst Characterization
The extensive characterization of the catalyst revealed that the introduction of iodide stabilizes unique copper sites (Cuδ⁺–Cu⁰) which enhance carbon-carbon coupling of *C2H3 intermediates, fostering the production of 1,3-butadiene. According to Professor Yeo, "This work is the culmination of an intense collaboration between experimentalists and theoreticians, along with our industrial partners, to uncover sustainable pathways for producing essential chemicals like 1,3-butadiene."
Future Directions
Looking ahead, the research team aims to build upon their findings to develop innovative catalysts capable of converting acetylene into longer-chain hydrocarbons. This advancement could open new avenues for sustainable aviation fuels, further supporting the industry's shift towards greener alternatives.
Conclusion
With the world increasingly moving towards sustainable practices, this groundbreaking research is expected to play a crucial role in reshaping the chemical manufacturing landscape—one that not only meets the demands of industry but also honors our planet. Could this be the key to eco-friendly rubber production? Only time will tell!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)